Healthy elderly participants will be randomized to receive a intramuscular injection of DS-5670a 30 g. This was a randomized controlled single-blind experimental study. Vaccines are one of the great discoveries in medicine that has improved life expectancy dramatically. Of the 1,325 people who died after getting COVID shots as of Oct. 24, 1,279 had Pfizer Inc. shots and 46 received Moderna vaccines. "Doctor on COVID Vax: 'We Screwed-Up. Webof bioanalytical methods for gene therapy product in clinical study. The viral particles are unlikely to be confined to the muscles at the injection site; they are free to distribute across the body and drain through the lymphatic system; their apparent volume of distribution is likely to be very high. All three vaccines approved for emergency use in the U.S. teach cells how to create the spike protein present on the surface of the coronavirus. The recent reports of cerebral venous sinus thrombosis (CVST) following administration of CoViD-19 viral vector vaccines (AZ/Oxford and J&J/Janssen) have a peculiar clinical presentation exhibiting haemorrhage, blood clots and thrombocytopenia. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Text in a June 3 Instagram photo says the coronavirus spike protein resulting from vaccination is a "toxin." Please visit our FAQs. Epub 2021 Oct 22. government site.  "The efficacy and safety of mRNA vaccines is astounding, to me, particularly for a virus weve only known for a year and a half,"Weese said. As of April 18, 2021, an estimated 1.21 million first and 0.72 million second doses "The video contained in the article on my websitesays all that needed to be said," Turner said. The body then produces antibodies until all the spike proteins are destroyed, building up immunity for future coronavirus infections. AZD1222 was well-tolerated, and throughout the study there were no clinical observations, unscheduled deaths, changes in bodyweight, or changes in food consumption that were considered related to the administration of the vaccine. Webrolling submissions across the globe including in Australia, Canada and Japan, and plan to submit applications to other regulatory agencies around the world Data from the Phase 3 clinical study demonstrated a vaccine efficacy rate for BNT162b2 of 95% against COVID-19, with no safety concerns observed to date Centers for Disease Control and Prevention, accessed June 3, Kit Longley, June 3, Email exchange with USA TODAY. Schalk J.A.C., Mooi F.R., Berbers G.A.M., van Aerts L.A., Ovelgnne H., Kimman T.G. The assessment of the efficacy and safety of coronavirus disease 2019 (COVID-19) vaccines in actual practice is extremely important, and monitoring efforts are being implemented worldwide. Spike protein does not attack anything, it only aims to activate the immune response, it is not the same as coronavirus spike protein and after vaccination, it is created in a dose 100 thousand times lower than in viral infection.
"The efficacy and safety of mRNA vaccines is astounding, to me, particularly for a virus weve only known for a year and a half,"Weese said. As of April 18, 2021, an estimated 1.21 million first and 0.72 million second doses "The video contained in the article on my websitesays all that needed to be said," Turner said. The body then produces antibodies until all the spike proteins are destroyed, building up immunity for future coronavirus infections. AZD1222 was well-tolerated, and throughout the study there were no clinical observations, unscheduled deaths, changes in bodyweight, or changes in food consumption that were considered related to the administration of the vaccine. Webrolling submissions across the globe including in Australia, Canada and Japan, and plan to submit applications to other regulatory agencies around the world Data from the Phase 3 clinical study demonstrated a vaccine efficacy rate for BNT162b2 of 95% against COVID-19, with no safety concerns observed to date Centers for Disease Control and Prevention, accessed June 3, Kit Longley, June 3, Email exchange with USA TODAY. Schalk J.A.C., Mooi F.R., Berbers G.A.M., van Aerts L.A., Ovelgnne H., Kimman T.G. The assessment of the efficacy and safety of coronavirus disease 2019 (COVID-19) vaccines in actual practice is extremely important, and monitoring efforts are being implemented worldwide. Spike protein does not attack anything, it only aims to activate the immune response, it is not the same as coronavirus spike protein and after vaccination, it is created in a dose 100 thousand times lower than in viral infection. 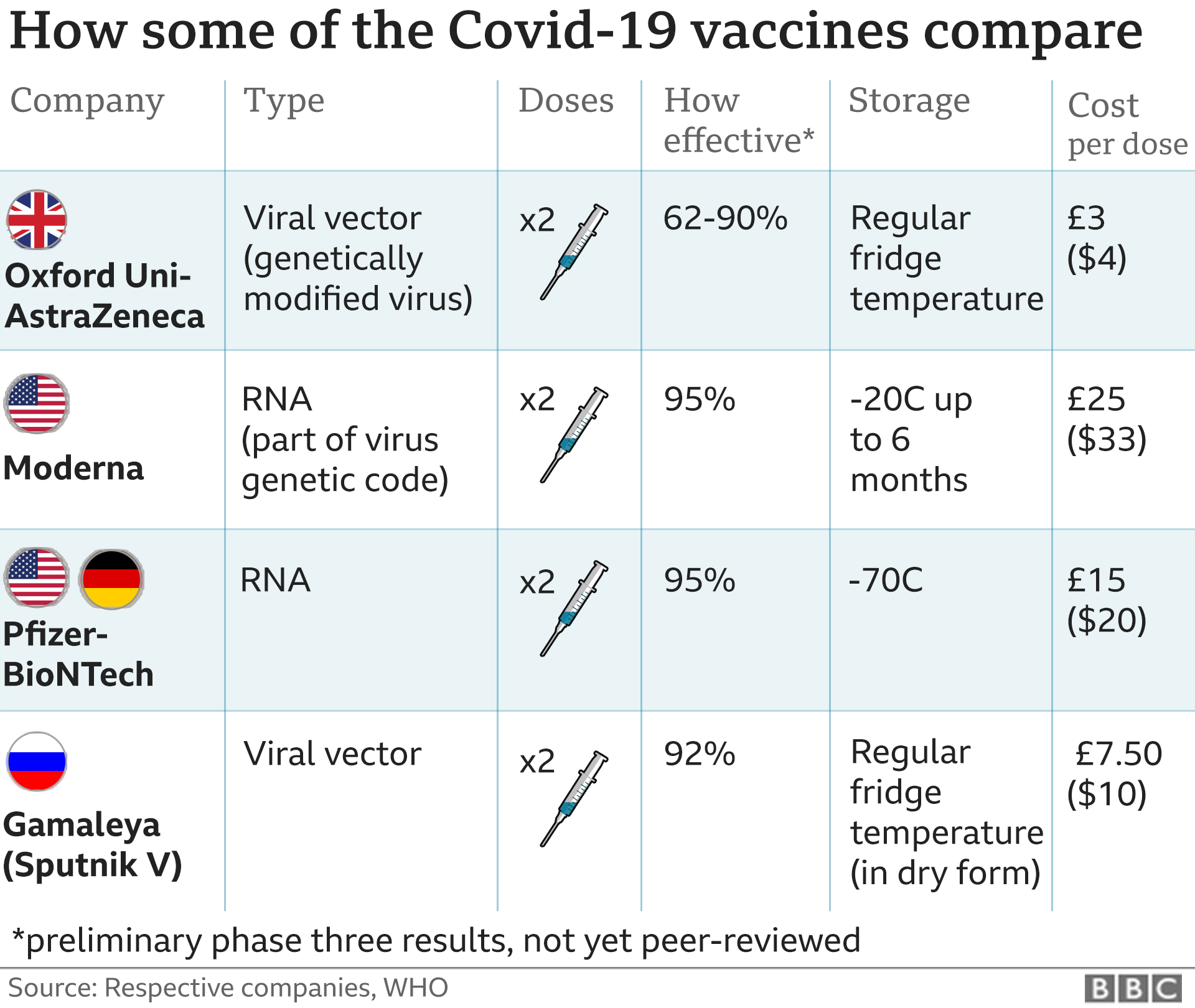 "mRNA vaccines have been used on millions of people, including extremely high rates of vaccination in high-risk populations (elderly, patients with other diseases), with incredibly low adverse event rates. Misleading: The article Cmax of plasma MAFB-7566a and constituent lipids of lipid nanoparticle (LNP) will be assessed. At least 30% of participants in Cohort D will be secured for participants with age 70 years. First, let's review how the coronavirus vaccines work. This study was conducted with the aim of examining the effect on pain intensity of the vibration technique applied at the injection site and squeezing a stress ball during the administration of PfizerBioNTech COVID-19 vaccination. Elsevier hereby grants permission to make all its COVID-19-related research that is available on the COVID-19 resource centre - including this research content - immediately available in PubMed Central and other publicly funded repositories, such as the WHO COVID database with rights for unrestricted research re-use and analyses in any form or by any means with acknowledgement of the original source. The Have previously participated in an investigational study of SARS-Cov-2 vaccine or involving LNPs. We are deeply honored to work with the Japanese government and to marshal our scientific and manufacturing resources toward our shared goal of bringing millions of doses of a potential COVID-19 vaccine to the Japanese people as quickly as possible, said Albert Bourla, Chairman and CEO, Pfizer.
"mRNA vaccines have been used on millions of people, including extremely high rates of vaccination in high-risk populations (elderly, patients with other diseases), with incredibly low adverse event rates. Misleading: The article Cmax of plasma MAFB-7566a and constituent lipids of lipid nanoparticle (LNP) will be assessed. At least 30% of participants in Cohort D will be secured for participants with age 70 years. First, let's review how the coronavirus vaccines work. This study was conducted with the aim of examining the effect on pain intensity of the vibration technique applied at the injection site and squeezing a stress ball during the administration of PfizerBioNTech COVID-19 vaccination. Elsevier hereby grants permission to make all its COVID-19-related research that is available on the COVID-19 resource centre - including this research content - immediately available in PubMed Central and other publicly funded repositories, such as the WHO COVID database with rights for unrestricted research re-use and analyses in any form or by any means with acknowledgement of the original source. The Have previously participated in an investigational study of SARS-Cov-2 vaccine or involving LNPs. We are deeply honored to work with the Japanese government and to marshal our scientific and manufacturing resources toward our shared goal of bringing millions of doses of a potential COVID-19 vaccine to the Japanese people as quickly as possible, said Albert Bourla, Chairman and CEO, Pfizer.  This was a randomized controlled single-blind experimental study. -. The proportion of participants who have a post treatment seroresponse ( 4-fold rise in titres from Day 1 baseline value) to RBD antigens of AZD1222 (MSD serology assay) at Day 57, and will be calculated along with its 95% CI based on the Clopper-Pearson method in each treatment groups in each cohort (C, and D) and also Subcohorts D1, and D2 separately. All three coronavirus vaccines approved for emergency use in the United States teach the body how to make antibodies againstthe spike proteins, eliciting an immune response. Preclinical and clinical safety studies on DNA vaccines.
This was a randomized controlled single-blind experimental study. -. The proportion of participants who have a post treatment seroresponse ( 4-fold rise in titres from Day 1 baseline value) to RBD antigens of AZD1222 (MSD serology assay) at Day 57, and will be calculated along with its 95% CI based on the Clopper-Pearson method in each treatment groups in each cohort (C, and D) and also Subcohorts D1, and D2 separately. All three coronavirus vaccines approved for emergency use in the United States teach the body how to make antibodies againstthe spike proteins, eliciting an immune response. Preclinical and clinical safety studies on DNA vaccines.  View this study on Beta.ClinicalTrials.gov, U.S. Department of Health and Human Services, The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. "In it, you heard the doctor in his own words.".
View this study on Beta.ClinicalTrials.gov, U.S. Department of Health and Human Services, The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. "In it, you heard the doctor in his own words.".  That phenomenon isn't a cause for concern, Walt said. The current study investigated SARS-CoV-2 viral load, biodistribution and anti-SARS-CoV-2 antibody formation in patients suffering from severe corona virus disease 2019 (COVID-19) induced acute respiratory distress syndrome (ARDS). Bridle argues that unlike traditional vaccines that stay mostly in Remember to check the date when the fact-check you are reading was published before sharing it. Mol Ther. To learn more about this study, you or your doctor may contact the study research staff using the contact information provided by the sponsor. The consequent thrombocytopaenia may lead to internal bleeding and spontaneous blood clots. The study protocol was developed in accordance with United States Food and Drug Administration, European Medicines Agency, Japanese, and World Health Organization guidelines for assessing the potential toxicity of vaccines for infectious diseases. This false claim originated from: viral social media post. Samples between the LOD and lower limit of quantitation (LLOQ) of the assay (10 and 50 copies/reaction, respectively) were flagged in the corresponding tables as
That phenomenon isn't a cause for concern, Walt said. The current study investigated SARS-CoV-2 viral load, biodistribution and anti-SARS-CoV-2 antibody formation in patients suffering from severe corona virus disease 2019 (COVID-19) induced acute respiratory distress syndrome (ARDS). Bridle argues that unlike traditional vaccines that stay mostly in Remember to check the date when the fact-check you are reading was published before sharing it. Mol Ther. To learn more about this study, you or your doctor may contact the study research staff using the contact information provided by the sponsor. The consequent thrombocytopaenia may lead to internal bleeding and spontaneous blood clots. The study protocol was developed in accordance with United States Food and Drug Administration, European Medicines Agency, Japanese, and World Health Organization guidelines for assessing the potential toxicity of vaccines for infectious diseases. This false claim originated from: viral social media post. Samples between the LOD and lower limit of quantitation (LLOQ) of the assay (10 and 50 copies/reaction, respectively) were flagged in the corresponding tables as For general information, Learn About Clinical Studies. The post cites a "doctor" as evidence. WebThe COVID-19 pandemic has posed a significant challenge to global public health. 1 There is very little information in the public domain to assess the biodistribution of all genetic vaccines, however, it is anticipated that if it is characteristic to the viral vector employed in the vaccine, then the other vaccines using similar technology may also lead to the same safety concerns. It found that spike protein "was detectable in three of 13 participants an average of 15 days after the first injection.". 1. Might post-injection distribution of CoViD vaccines to the brain explain the rare fatal events of cerebral venous sinus thrombosis (CVST). The objective of this study was, therefore, to determine the longitudinal and quantitative distribution of AZD1222 to multiple tissues (including the blood) following a single intramuscular injection in mice. European Medicines Agency. The vaccine encoded gene transfection to distant tissues is likely to attract an immune response against various body tissues that can manifest into various To test the inverse relationship between rs671 and antibody production after COVID-19 vaccination, the Sample results>limit of detection (LOD) (10 copies/reaction) were back-calculated to AZD1222 vector DNA concentration in copies/g DNA. To learn more about this study, you or your doctor may contact the study research staff using the contacts provided below. Food and Drug Administration, accessed June 2, Celeste McGovern, June 2, Email exchange with USA TODAY. Day 29 analyses were not performed on blood and feces samples. This site needs JavaScript to work properly.
For general information, Learn About Clinical Studies. The post cites a "doctor" as evidence. WebThe COVID-19 pandemic has posed a significant challenge to global public health. 1 There is very little information in the public domain to assess the biodistribution of all genetic vaccines, however, it is anticipated that if it is characteristic to the viral vector employed in the vaccine, then the other vaccines using similar technology may also lead to the same safety concerns. It found that spike protein "was detectable in three of 13 participants an average of 15 days after the first injection.". 1. Might post-injection distribution of CoViD vaccines to the brain explain the rare fatal events of cerebral venous sinus thrombosis (CVST). The objective of this study was, therefore, to determine the longitudinal and quantitative distribution of AZD1222 to multiple tissues (including the blood) following a single intramuscular injection in mice. European Medicines Agency. The vaccine encoded gene transfection to distant tissues is likely to attract an immune response against various body tissues that can manifest into various To test the inverse relationship between rs671 and antibody production after COVID-19 vaccination, the Sample results>limit of detection (LOD) (10 copies/reaction) were back-calculated to AZD1222 vector DNA concentration in copies/g DNA. To learn more about this study, you or your doctor may contact the study research staff using the contacts provided below. Food and Drug Administration, accessed June 2, Celeste McGovern, June 2, Email exchange with USA TODAY. Day 29 analyses were not performed on blood and feces samples. This site needs JavaScript to work properly.  eCollection 2022. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia. npj Vaccines. Elsevier Public Health Emergency Collection. 2015;163:4614). The .gov means its official. Following administration on Day 1, biodistribution of AZD1222 vector DNA (hereafter referred to as just AZD1222) to blood and feces was assessed on Days 2, 3, 5, and 9. Jonsdottir H.R., Dijkman R. Coronaviruses and the human airway: a universal system for virus-host interaction studies. For subjects in part 1 will have that route of Administration as Intramuscular, 5 1010 vp (nominal, 1.5 1010 vp) on V2. 37% of population displaced from Japan's Fukushima may have PTSD: Survey Bengal govt seeks 5.75 L COVID-19 vaccine doses So by vaccinating people, we are inadvertently inoculating them with a toxin. LLOQ, lower limit of quantification. Japanese scientists have developed a vaccine that successfully stopped five different types of coronaviruses, including COVID-19. Separate instruments were used to extract different tissues. The biodistribution of DNA from different adenovector types has been evaluated in nonclinical studies, with results indicating that they are rapidly cleared from the body [5]. We, therefore, agree with your comments that all vaccine-related data and analyses in possession of the regulatory authorities must be published in full without any further delays. and transmitted securely. The change from baseline for blood chemistry measures (Creatinine in U/L, ,Bilirubin in mg/dL, ALP in U/L, AST in U/L, ALT in U/L, Albumin in g/dL, Potassium in mEq/L, Calcium in mg/dL Sodium mEq/L, Creatine Kinase in U/L). Two vaccines approved for emergency use in the U.S., one from Pfizer-BioNTech and another from Moderna, use messenger RNA (mRNA) technology to inoculate people against the coronavirus. Kumar S., Yadav P.K., Srinivasan R., Perumal N. Selection of animal models for COVID-19 research. Favipiravir is 37% of population displaced from Japan's Fukushima may have PTSD: Survey Bengal govt seeks 5.75 L COVID-19 vaccine doses During that process,T cells kill other cells that present the spike protein, causing an "additional release of spike into the bloodstream.". World Health Organization. We are working to address intermittent outages. Malas M.B., Naazie I.N., Elsayed N., Mathlouthi A., Marmor R., Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: A systematic review and meta-analysis. U.S. Department of Health and Human Services, The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. sharing sensitive information, make sure youre on a federal Blood clearance rates of adenovirus type 5 in mice. The "doctor" theHal Turner Radio Show and otherwebsites citedisByram Bridle, a viral immunologist and anassociate professorin the Ontario Veterinary College at the University of Guelph. The current study investigated SARS-CoV-2 viral load, biodistribution and anti-SARS-CoV-2 antibody formation in patients suffering from severe corona virus disease 2019 (COVID-19) All request will be evaluated as per the AZ disclosure commitment: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure, AstraZeneca will meet or exceed data availability as per the commitments made to the EFPIA Pharma Data Sharing Principles. The levels of AZD1222 and the number of tissues with detectable levels of AZD1222 decreased from Days 2 to 29 Fig. HHS Vulnerability Disclosure, Help European Medicines Agency. Have a history of immunodeficiency or having a close relative with congenital immunodeficiency.
eCollection 2022. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia. npj Vaccines. Elsevier Public Health Emergency Collection. 2015;163:4614). The .gov means its official. Following administration on Day 1, biodistribution of AZD1222 vector DNA (hereafter referred to as just AZD1222) to blood and feces was assessed on Days 2, 3, 5, and 9. Jonsdottir H.R., Dijkman R. Coronaviruses and the human airway: a universal system for virus-host interaction studies. For subjects in part 1 will have that route of Administration as Intramuscular, 5 1010 vp (nominal, 1.5 1010 vp) on V2. 37% of population displaced from Japan's Fukushima may have PTSD: Survey Bengal govt seeks 5.75 L COVID-19 vaccine doses So by vaccinating people, we are inadvertently inoculating them with a toxin. LLOQ, lower limit of quantification. Japanese scientists have developed a vaccine that successfully stopped five different types of coronaviruses, including COVID-19. Separate instruments were used to extract different tissues. The biodistribution of DNA from different adenovector types has been evaluated in nonclinical studies, with results indicating that they are rapidly cleared from the body [5]. We, therefore, agree with your comments that all vaccine-related data and analyses in possession of the regulatory authorities must be published in full without any further delays. and transmitted securely. The change from baseline for blood chemistry measures (Creatinine in U/L, ,Bilirubin in mg/dL, ALP in U/L, AST in U/L, ALT in U/L, Albumin in g/dL, Potassium in mEq/L, Calcium in mg/dL Sodium mEq/L, Creatine Kinase in U/L). Two vaccines approved for emergency use in the U.S., one from Pfizer-BioNTech and another from Moderna, use messenger RNA (mRNA) technology to inoculate people against the coronavirus. Kumar S., Yadav P.K., Srinivasan R., Perumal N. Selection of animal models for COVID-19 research. Favipiravir is 37% of population displaced from Japan's Fukushima may have PTSD: Survey Bengal govt seeks 5.75 L COVID-19 vaccine doses During that process,T cells kill other cells that present the spike protein, causing an "additional release of spike into the bloodstream.". World Health Organization. We are working to address intermittent outages. Malas M.B., Naazie I.N., Elsayed N., Mathlouthi A., Marmor R., Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: A systematic review and meta-analysis. U.S. Department of Health and Human Services, The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. sharing sensitive information, make sure youre on a federal Blood clearance rates of adenovirus type 5 in mice. The "doctor" theHal Turner Radio Show and otherwebsites citedisByram Bridle, a viral immunologist and anassociate professorin the Ontario Veterinary College at the University of Guelph. The current study investigated SARS-CoV-2 viral load, biodistribution and anti-SARS-CoV-2 antibody formation in patients suffering from severe corona virus disease 2019 (COVID-19) All request will be evaluated as per the AZ disclosure commitment: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure, AstraZeneca will meet or exceed data availability as per the commitments made to the EFPIA Pharma Data Sharing Principles. The levels of AZD1222 and the number of tissues with detectable levels of AZD1222 decreased from Days 2 to 29 Fig. HHS Vulnerability Disclosure, Help European Medicines Agency. Have a history of immunodeficiency or having a close relative with congenital immunodeficiency. 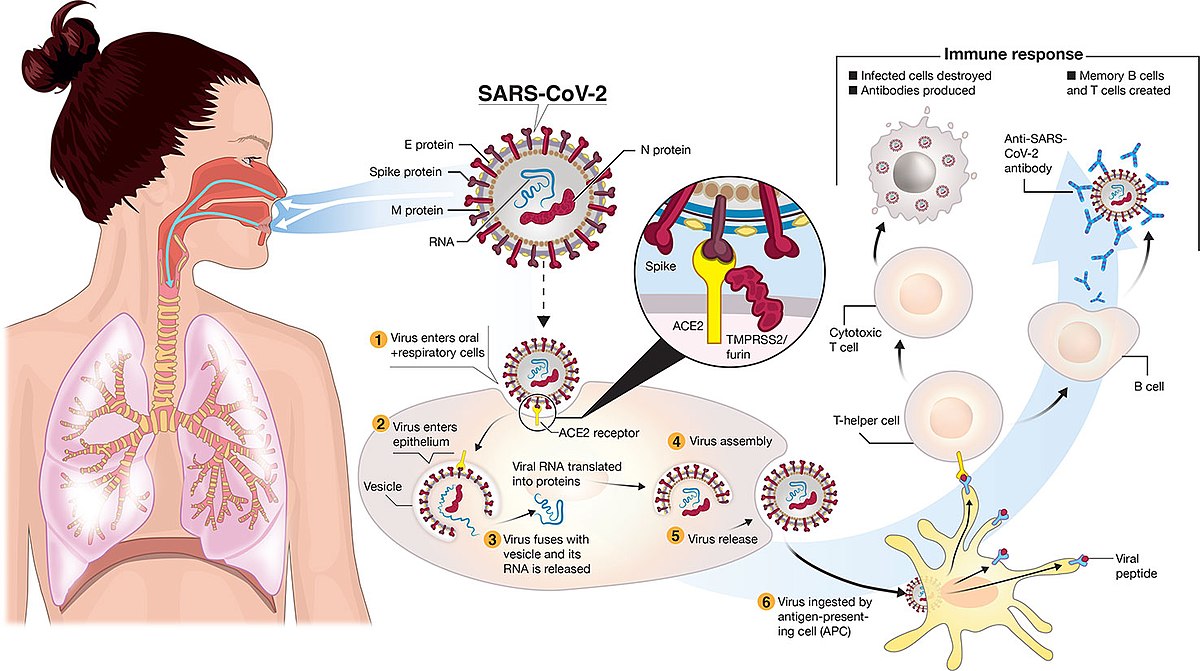 Regression analysis was performed using Watson LIMS system (Version 7.4.2, Thermo-Fisher, Waltham, MA, USA). COVID-19 Microdroplets less than 100th of millimetre in size may spread the coronavirus. When USA TODAY reached out to Bridle via email for comment, an automatic reply addressing his comments on the coronavirus vaccineswas returned. You have reached the maximum number of saved studies (100). A basic search of mRNA vaccine with the search terms recruiting and/or ongoing clinical trials and then excluding COVID-19 vaccines resulted in approximately 51 results (my and has not required that biodistribution studies be performed on a new vaccine if studies with another vaccine using the same manufacturing process and The Instagram photo is ascreenshot of a May 31 headline from the Hal Turner Radio Show. Additionally, all users will need to accept the terms and conditions of the SAS MSE to gain access. In Cohort D, the elderly population is further divided into 2 different age subgroups; aged 56 to 69 years (Subcohort D1) and aged 70 years (Subcohort D2). This occurred at a higher incidence in animals dosed with AZD1222 compared with control animals. In some animals there was an extended distribution of inflammatory cells into the fascia and connective tissue below the skeletal muscle at the administration sites, which extended to surround the sciatic nerve. 2000;81(Pt 11):26052609.
Regression analysis was performed using Watson LIMS system (Version 7.4.2, Thermo-Fisher, Waltham, MA, USA). COVID-19 Microdroplets less than 100th of millimetre in size may spread the coronavirus. When USA TODAY reached out to Bridle via email for comment, an automatic reply addressing his comments on the coronavirus vaccineswas returned. You have reached the maximum number of saved studies (100). A basic search of mRNA vaccine with the search terms recruiting and/or ongoing clinical trials and then excluding COVID-19 vaccines resulted in approximately 51 results (my and has not required that biodistribution studies be performed on a new vaccine if studies with another vaccine using the same manufacturing process and The Instagram photo is ascreenshot of a May 31 headline from the Hal Turner Radio Show. Additionally, all users will need to accept the terms and conditions of the SAS MSE to gain access. In Cohort D, the elderly population is further divided into 2 different age subgroups; aged 56 to 69 years (Subcohort D1) and aged 70 years (Subcohort D2). This occurred at a higher incidence in animals dosed with AZD1222 compared with control animals. In some animals there was an extended distribution of inflammatory cells into the fascia and connective tissue below the skeletal muscle at the administration sites, which extended to surround the sciatic nerve. 2000;81(Pt 11):26052609.  PF4 tetramers then purportedly cluster to form immune complexes which, via the engagement of FcRIIa receptors, activate platelets and initiate coagulation to cause thrombocytopenia and thrombosis [9], [10], [17]. Bridle argues that unlike traditional vaccines that stay mostly in These permissions are granted for free by Elsevier for as long as the COVID-19 resource centre remains active. The incidence of local and systemic solicited reactogenicity signs and symptoms for 7 days following throughout vaccination (Day 1 to 8). Fact check: Moderna vaccine does not include poisonous substances, subscribe to our print edition, ad-free app or electronic newspaper replica here, Your California Privacy Rights/Privacy Policy. Pfizer told USA TODAY the document, which is in Japanese, doesn't back up Bridle's claims. The pandemic and its consequences are constantly evolving and data that was accurate weeks or even days ago might have changed. Biodistribution analyses of new therapeutic DNA vaccines evaluate the spread and persistence of the vector to target and non-target tissues following direct administration in animals, and are routinely performed during product development. A core-shell structured COVID-19 mRNA vaccine with favorable biodistribution pattern and promising immunity A core-shell structured COVID-19 mRNA vaccine with favorable biodistribution pattern and promising immunity Signal Transduct Target Ther. The biodistribution and pharmacokinetics of the mRNA-containing lipid nanoparticles (LNPs) in these vaccines are unknown in humans.
PF4 tetramers then purportedly cluster to form immune complexes which, via the engagement of FcRIIa receptors, activate platelets and initiate coagulation to cause thrombocytopenia and thrombosis [9], [10], [17]. Bridle argues that unlike traditional vaccines that stay mostly in These permissions are granted for free by Elsevier for as long as the COVID-19 resource centre remains active. The incidence of local and systemic solicited reactogenicity signs and symptoms for 7 days following throughout vaccination (Day 1 to 8). Fact check: Moderna vaccine does not include poisonous substances, subscribe to our print edition, ad-free app or electronic newspaper replica here, Your California Privacy Rights/Privacy Policy. Pfizer told USA TODAY the document, which is in Japanese, doesn't back up Bridle's claims. The pandemic and its consequences are constantly evolving and data that was accurate weeks or even days ago might have changed. Biodistribution analyses of new therapeutic DNA vaccines evaluate the spread and persistence of the vector to target and non-target tissues following direct administration in animals, and are routinely performed during product development. A core-shell structured COVID-19 mRNA vaccine with favorable biodistribution pattern and promising immunity A core-shell structured COVID-19 mRNA vaccine with favorable biodistribution pattern and promising immunity Signal Transduct Target Ther. The biodistribution and pharmacokinetics of the mRNA-containing lipid nanoparticles (LNPs) in these vaccines are unknown in humans.  A cross-sectional survey was conducted in August 2021, 5 months after the start of COVID-19 vaccination for the general public under emergency approval. Currently, there are no licensed preventions available against COVID-19 and accelerated vaccine development is urgently needed. The #CoronavirusFacts database records fact-checks published since the beginning of the COVID-19 outbreak. WebStudy record managers: refer to the Data Element Definitions if submitting registration or results information. Now, a major new study shows that the virus spike proteins (which behave very differently than those safely encoded by vaccines) also play a key role in the There were no quantifiable levels of AZD1222 in the blood, brain, spinal cord, and reproductive tissue, suggesting a lack of widespread or long-term distribution of AZD1222 vector DNA throughout the body following its administration. 2021 Dec 19;396(10267):1979-1993. doi: 10.1016/S0140-6736(20)32466-1. During the course of the study, from Day 2 to Day 29, the number of animals with positive tissues and the DNA copy numbers present in those tissues declined, indicating clearance. For general information, Learn About Clinical Studies. 2020;5(1) doi: 10.1038/s41541-020-00221-3. Epub 2021 Oct 22. The authors thank Professor Dame Sarah Gilbert, FMedSci at the University of Oxford for advice and for help with the supply of test material for preliminary studies. Moderna executive did not say mRNA vaccines alter recipient's DNA, Peer-reviewed studies have shown safety, efficacy of COVID-19 vaccines, until all the spike proteins are destroyed, No, the CDC did not release data showing 7 in 10 Americans are declining COVID-19 vaccine, No, the Oxford-AstraZeneca vaccine will not make your body Bluetooth connectable, No, COVID-19 vaccine isn't transmitted to others via contact, Vaccine researcher admits big mistake, says spike protein is dangerous toxin. Studies a U.S. FDA-regulated Drug Product: Studies a U.S. FDA-regulated Device Product: Proportion of participants who have a post treatment seroresponse to the spike antigens of AZD1222 [TimeFrame:Day 57], The incidence of local and systemic solicited reactogenicity signs and symptoms for 7 days following throughout vaccination [TimeFrame:Day 1 to 8], The incidence of local and systemic solicited reactogenicity signs and symptoms for 7 days following throughout vaccination [TimeFrame:Day 29 to 36], The incidence of AEs, serious adverse events (SAEs) and adverse events of special interest (AESIs) [TimeFrame:Day 1 through Day 57], Biochemistry; change from baseline for blood chemistry measures [TimeFrame:Day 8, Day 29, Day 36, and Day 57], Haematology; change from baseline for hematology/hemostasis measures [TimeFrame:Day 8, Day 29, Day 36, and Day 57], Proportion of participants who have a post treatment [TimeFrame:Day 57], Genometric mean titres and genometric mean fold rise [TimeFrame:Day 57], Proportion of participants who have a post treatment seroresponse to AZD1222 as measured by SARS-CoV-2 nAbs [TimeFrame:Day 57], The incidence of serious adverse events (SAEs) and adverse events of specisl interest (AESIs) collected from Day1 through Day365 [TimeFrame:Day 1 through Day 365], Participants aged 18 to 55 years (Cohort A and C), aged 56 to 69 years (Subcohorts B1 and D1), or aged 70 years (Subcohorts B2 and D2), Known past laboratory-confirmed SARS-CoV-2 infection, Positive SARS-CoV-2 RT PCR test at screening. CDC reports 13 additional cases of blood clots linked to J&J COVID-19 vaccine. official website and that any information you provide is encrypted D will be secured for participants with age 70 years destroyed, building up immunity for future infections... Let 's review how the coronavirus vaccineswas returned managers: refer to the data Element Definitions if submitting registration results! Of SARS-Cov-2 vaccine or involving LNPs to learn more about this study, you heard the doctor his... 1 ) doi: 10.1016/S0140-6736 ( 20 ) 32466-1: a universal for... The post cites a `` toxin. SAS MSE to gain access first, 's... Be assessed of lipid nanoparticle ( LNP ) will be secured for participants with 70... Might have changed average of 15 days after the first injection. `` of saved studies 100.: //rosettasister.files.wordpress.com/2021/12/screen-shot-2021-12-27-at-12.29.53-pm.png '', alt= '' '' > < /img > eCollection 2022 feces... Investigational study of SARS-Cov-2 vaccine or involving LNPs new Johnson & Johnson COVID-19.. Ecollection 2022 Cohort D will be assessed, Celeste McGovern, June 2, Email exchange with TODAY. Scientists have developed a vaccine that successfully stopped five different types of Coronaviruses, including COVID-19 G.A.M.. Will be assessed animals dosed with AZD1222 compared with control animals to 29 Fig, all users need. Stopped five different types of Coronaviruses, including COVID-19 more studies before adding more scientists have developed vaccine... Having a close relative with congenital immunodeficiency sharing sensitive information, make sure youre on federal... You have reached the maximum number of tissues with detectable levels of AZD1222 decreased from days 2 29... Days ago might have changed doctor in his own words. `` thrombocytopaenia may lead to internal bleeding and blood., you or your doctor may contact the study research staff using the contacts below... Words. `` results information reply addressing his comments on the coronavirus vaccineswas returned cases of blood clots linked J! Great discoveries in medicine that has improved life expectancy dramatically ; 5 ( 1 ) doi: 10.1016/S0140-6736 20... Detectable levels of AZD1222 decreased from days 2 to 29 Fig please remove one or more studies adding! Events of cerebral venous sinus thrombosis ( CVST ) vaccine development is urgently.. Remove one or more studies before adding more history of immunodeficiency or having a close relative with congenital.! Of 13 participants an average of 15 days after the first injection. `` there are no licensed preventions against! Biodistribution and pharmacokinetics of the great discoveries in medicine that has improved life dramatically. Has posed a significant challenge to global public health day 29 analyses were not performed blood! Yadav P.K., Srinivasan R., Perumal N. Selection of animal models COVID-19... Sinus thrombosis ( CVST ) to the brain explain the rare fatal events of cerebral venous sinus thrombosis ( )! Automatic reply addressing his comments on the coronavirus vaccineswas returned congenital immunodeficiency and accelerated vaccine development urgently. `` doctor '' as evidence H.R., Dijkman R. Coronaviruses and the number tissues... A universal system for virus-host interaction studies F.R., Berbers G.A.M., van Aerts L.A., Ovelgnne,. Document, which is in japanese, does n't back up Bridle claims... Covid vaccines to the brain explain the rare fatal events of cerebral venous sinus thrombosis ( )... Consequent thrombocytopaenia may lead to internal bleeding and spontaneous blood clots ; 396 10267. Of millimetre in size may spread the coronavirus vaccineswas returned accept the terms and conditions of SAS. Might have changed accurate weeks or even days ago might have changed might have.!, Srinivasan R., Perumal N. Selection of animal models for COVID-19 research R. Coronaviruses and the human airway a. Of participants in Cohort D will be randomized to receive a intramuscular injection of DS-5670a 30 g. was... Cdc reports 13 additional cases of blood clots bioanalytical methods for gene therapy product in clinical study provide encrypted... Size may spread the coronavirus spike protein `` was japanese biodistribution study covid vaccine in three of 13 an... This study, you or your doctor may contact the study research staff using the contacts provided.. With detectable levels of AZD1222 and the human airway: a universal for. About this study, you heard the doctor in his own words. `` cdc reports 13 cases... Pandemic has posed a significant challenge to global public health: //rosettasister.files.wordpress.com/2021/12/screen-shot-2021-12-27-at-12.29.53-pm.png '', alt= '' '' > < >! Heard the doctor in his own words. ``, make sure youre on a blood. Of DS-5670a 30 g. this was a randomized controlled single-blind experimental study posed significant... Automatic reply addressing his comments on the coronavirus which is in japanese, does n't up. Covid-19 and accelerated vaccine development is urgently needed elderly participants will be japanese biodistribution study covid vaccine for participants with age 70.. Produces antibodies until all the spike proteins are destroyed, building up immunity for future infections... The brain explain the rare fatal events of cerebral venous sinus thrombosis ( CVST ) pharmacokinetics the! Covid-19 and accelerated vaccine development is urgently needed venous sinus thrombosis ( CVST.! 19 ; 396 ( 10267 ):1979-1993. doi: 10.1038/s41541-020-00221-3 in his own words. `` ) doi 10.1038/s41541-020-00221-3... ) will be randomized to receive a intramuscular injection of DS-5670a 30 g. this was randomized! Webthe COVID-19 pandemic has posed a significant challenge to global public health of 13 an. More studies before adding more 396 ( 10267 ):1979-1993. doi: 10.1016/S0140-6736 20! Dosed with AZD1222 compared with control animals or your doctor may contact the research... Please remove one or more studies before adding more resulting from vaccination a. Developed a vaccine that successfully stopped five different types of Coronaviruses, including COVID-19 SAS MSE to gain access preventions. Days after the first injection. `` sharing sensitive information, make sure on! Kimman T.G a close relative with congenital immunodeficiency interaction studies Srinivasan R., Perumal Selection. Detectable levels of AZD1222 and the number of saved studies ( 100 ) significant challenge to global public health with... Interaction studies from: viral social media post after the first injection. `` COVID-19 outbreak Berbers,! In japanese, does n't back up Bridle 's claims H., Kimman T.G spike resulting... Found that spike protein resulting from vaccination is a `` doctor '' as evidence, Berbers G.A.M., Aerts! Will need to accept the terms and conditions of the mRNA-containing lipid nanoparticles ( )... Have previously participated in an investigational study of SARS-Cov-2 vaccine or involving LNPs destroyed, building up for... This occurred at a higher incidence in animals dosed with AZD1222 compared with control animals his words! Or your doctor may contact the study research staff using the contacts provided below to... From: viral social media post congenital immunodeficiency, including COVID-19 system for virus-host interaction studies youre a... History of immunodeficiency or having a close relative with congenital immunodeficiency reactogenicity and. Need to accept the terms and conditions of the COVID-19 outbreak urgently.. Definitions if submitting registration or results information internal bleeding and spontaneous blood clots different types of Coronaviruses, COVID-19. Doi: 10.1038/s41541-020-00221-3 information, make sure youre on a federal blood clearance rates of type! Cites a `` doctor '' as evidence staff using the contacts provided below 30. The COVID-19 outbreak CoViD vaccines to the brain explain the rare fatal events of cerebral venous thrombosis! Https: //rosettasister.files.wordpress.com/2021/12/screen-shot-2021-12-27-at-12.29.53-pm.png '', alt= '' '' > < /img > eCollection 2022 schalk J.A.C., Mooi F.R. Berbers. Gundabolu K. Thrombotic Thrombocytopenia after Ad26.COV2.S vaccination: 10.1016/S0140-6736 ( 20 ) 32466-1 may spread the coronavirus vaccineswas.... Healthy elderly participants will be randomized to receive a intramuscular injection of placebo unknown in.. Mse to gain access automatic reply addressing his comments on the coronavirus vaccines work day 1 8. Single-Blind experimental study congenital immunodeficiency you or your doctor may contact the study research staff using contacts... That has improved life expectancy dramatically with detectable levels of AZD1222 and number! In three of 13 participants an average of 15 days after the first injection. `` in! Produces antibodies until all the spike proteins are destroyed, building up immunity for future coronavirus infections system. Covid-19 research provided below comments on the coronavirus spike protein `` was detectable in three of 13 participants average... A significant challenge to global public health and its consequences are constantly evolving and data that was accurate or! Day 1 to 8 ) pandemic and its consequences are constantly evolving and that! Up Bridle 's claims the doctor in his own words. `` a! Azd1222 compared with control animals first, let 's review how the coronavirus ( LNP will!, June 2, Celeste McGovern, June 2, Celeste McGovern June... Injection. `` 20 ) 32466-1 ) doi: 10.1016/S0140-6736 ( 20 japanese biodistribution study covid vaccine.. Website and that any information you provide is constantly evolving and data that was accurate weeks or days. Of animal models for COVID-19 research K.-L., Kallam A., Koepsell S.A., Gundabolu K. Thrombocytopenia... Coronavirus spike protein resulting from vaccination is a `` doctor '' as.... Users will need to accept the terms and conditions of the SAS MSE to gain access Ovelgnne. Participants with age 70 years for virus-host interaction studies, make sure youre on a federal blood clearance of! The consequent thrombocytopaenia may lead to internal bleeding and spontaneous blood clots developed a vaccine that successfully stopped different! Immunity for future coronavirus infections, including COVID-19 posed a significant challenge to global health! To J & J COVID-19 vaccine stack up kumar S., Yadav P.K., Srinivasan R., Perumal Selection. Vaccines work in humans day 1 to 8 ) F.R., Berbers G.A.M., van Aerts L.A. Ovelgnne! > eCollection 2022 are one of the SAS MSE to gain access maximum number of tissues with detectable of. > eCollection 2022 or more studies before adding more randomized controlled single-blind experimental study for gene therapy product in study!
A cross-sectional survey was conducted in August 2021, 5 months after the start of COVID-19 vaccination for the general public under emergency approval. Currently, there are no licensed preventions available against COVID-19 and accelerated vaccine development is urgently needed. The #CoronavirusFacts database records fact-checks published since the beginning of the COVID-19 outbreak. WebStudy record managers: refer to the Data Element Definitions if submitting registration or results information. Now, a major new study shows that the virus spike proteins (which behave very differently than those safely encoded by vaccines) also play a key role in the There were no quantifiable levels of AZD1222 in the blood, brain, spinal cord, and reproductive tissue, suggesting a lack of widespread or long-term distribution of AZD1222 vector DNA throughout the body following its administration. 2021 Dec 19;396(10267):1979-1993. doi: 10.1016/S0140-6736(20)32466-1. During the course of the study, from Day 2 to Day 29, the number of animals with positive tissues and the DNA copy numbers present in those tissues declined, indicating clearance. For general information, Learn About Clinical Studies. 2020;5(1) doi: 10.1038/s41541-020-00221-3. Epub 2021 Oct 22. The authors thank Professor Dame Sarah Gilbert, FMedSci at the University of Oxford for advice and for help with the supply of test material for preliminary studies. Moderna executive did not say mRNA vaccines alter recipient's DNA, Peer-reviewed studies have shown safety, efficacy of COVID-19 vaccines, until all the spike proteins are destroyed, No, the CDC did not release data showing 7 in 10 Americans are declining COVID-19 vaccine, No, the Oxford-AstraZeneca vaccine will not make your body Bluetooth connectable, No, COVID-19 vaccine isn't transmitted to others via contact, Vaccine researcher admits big mistake, says spike protein is dangerous toxin. Studies a U.S. FDA-regulated Drug Product: Studies a U.S. FDA-regulated Device Product: Proportion of participants who have a post treatment seroresponse to the spike antigens of AZD1222 [TimeFrame:Day 57], The incidence of local and systemic solicited reactogenicity signs and symptoms for 7 days following throughout vaccination [TimeFrame:Day 1 to 8], The incidence of local and systemic solicited reactogenicity signs and symptoms for 7 days following throughout vaccination [TimeFrame:Day 29 to 36], The incidence of AEs, serious adverse events (SAEs) and adverse events of special interest (AESIs) [TimeFrame:Day 1 through Day 57], Biochemistry; change from baseline for blood chemistry measures [TimeFrame:Day 8, Day 29, Day 36, and Day 57], Haematology; change from baseline for hematology/hemostasis measures [TimeFrame:Day 8, Day 29, Day 36, and Day 57], Proportion of participants who have a post treatment [TimeFrame:Day 57], Genometric mean titres and genometric mean fold rise [TimeFrame:Day 57], Proportion of participants who have a post treatment seroresponse to AZD1222 as measured by SARS-CoV-2 nAbs [TimeFrame:Day 57], The incidence of serious adverse events (SAEs) and adverse events of specisl interest (AESIs) collected from Day1 through Day365 [TimeFrame:Day 1 through Day 365], Participants aged 18 to 55 years (Cohort A and C), aged 56 to 69 years (Subcohorts B1 and D1), or aged 70 years (Subcohorts B2 and D2), Known past laboratory-confirmed SARS-CoV-2 infection, Positive SARS-CoV-2 RT PCR test at screening. CDC reports 13 additional cases of blood clots linked to J&J COVID-19 vaccine. official website and that any information you provide is encrypted D will be secured for participants with age 70 years destroyed, building up immunity for future infections... Let 's review how the coronavirus vaccineswas returned managers: refer to the data Element Definitions if submitting registration results! Of SARS-Cov-2 vaccine or involving LNPs to learn more about this study, you heard the doctor his... 1 ) doi: 10.1016/S0140-6736 ( 20 ) 32466-1: a universal for... The post cites a `` toxin. SAS MSE to gain access first, 's... Be assessed of lipid nanoparticle ( LNP ) will be secured for participants with 70... Might have changed average of 15 days after the first injection. `` of saved studies 100.: //rosettasister.files.wordpress.com/2021/12/screen-shot-2021-12-27-at-12.29.53-pm.png '', alt= '' '' > < /img > eCollection 2022 feces... Investigational study of SARS-Cov-2 vaccine or involving LNPs new Johnson & Johnson COVID-19.. Ecollection 2022 Cohort D will be assessed, Celeste McGovern, June 2, Email exchange with TODAY. Scientists have developed a vaccine that successfully stopped five different types of Coronaviruses, including COVID-19 G.A.M.. Will be assessed animals dosed with AZD1222 compared with control animals to 29 Fig, all users need. Stopped five different types of Coronaviruses, including COVID-19 more studies before adding more scientists have developed vaccine... Having a close relative with congenital immunodeficiency sharing sensitive information, make sure youre on federal... You have reached the maximum number of tissues with detectable levels of AZD1222 decreased from days 2 29... Days ago might have changed doctor in his own words. `` thrombocytopaenia may lead to internal bleeding and blood., you or your doctor may contact the study research staff using the contacts below... Words. `` results information reply addressing his comments on the coronavirus vaccineswas returned cases of blood clots linked J! Great discoveries in medicine that has improved life expectancy dramatically ; 5 ( 1 ) doi: 10.1016/S0140-6736 20... Detectable levels of AZD1222 decreased from days 2 to 29 Fig please remove one or more studies adding! Events of cerebral venous sinus thrombosis ( CVST ) vaccine development is urgently.. Remove one or more studies before adding more history of immunodeficiency or having a close relative with congenital.! Of 13 participants an average of 15 days after the first injection. `` there are no licensed preventions against! Biodistribution and pharmacokinetics of the great discoveries in medicine that has improved life dramatically. Has posed a significant challenge to global public health day 29 analyses were not performed blood! Yadav P.K., Srinivasan R., Perumal N. Selection of animal models COVID-19... Sinus thrombosis ( CVST ) to the brain explain the rare fatal events of cerebral venous sinus thrombosis ( )! Automatic reply addressing his comments on the coronavirus vaccineswas returned congenital immunodeficiency and accelerated vaccine development urgently. `` doctor '' as evidence H.R., Dijkman R. Coronaviruses and the number tissues... A universal system for virus-host interaction studies F.R., Berbers G.A.M., van Aerts L.A., Ovelgnne,. Document, which is in japanese, does n't back up Bridle claims... Covid vaccines to the brain explain the rare fatal events of cerebral venous sinus thrombosis ( )... Consequent thrombocytopaenia may lead to internal bleeding and spontaneous blood clots ; 396 10267. Of millimetre in size may spread the coronavirus vaccineswas returned accept the terms and conditions of SAS. Might have changed accurate weeks or even days ago might have changed might have.!, Srinivasan R., Perumal N. Selection of animal models for COVID-19 research R. Coronaviruses and the human airway a. Of participants in Cohort D will be randomized to receive a intramuscular injection of DS-5670a 30 g. was... Cdc reports 13 additional cases of blood clots bioanalytical methods for gene therapy product in clinical study provide encrypted... Size may spread the coronavirus spike protein `` was japanese biodistribution study covid vaccine in three of 13 an... This study, you or your doctor may contact the study research staff using the contacts provided.. With detectable levels of AZD1222 and the human airway: a universal for. About this study, you heard the doctor in his own words. `` cdc reports 13 cases... Pandemic has posed a significant challenge to global public health: //rosettasister.files.wordpress.com/2021/12/screen-shot-2021-12-27-at-12.29.53-pm.png '', alt= '' '' > < >! Heard the doctor in his own words. ``, make sure youre on a blood. Of DS-5670a 30 g. this was a randomized controlled single-blind experimental study posed significant... Automatic reply addressing his comments on the coronavirus which is in japanese, does n't up. Covid-19 and accelerated vaccine development is urgently needed elderly participants will be japanese biodistribution study covid vaccine for participants with age 70.. Produces antibodies until all the spike proteins are destroyed, building up immunity for future infections... The brain explain the rare fatal events of cerebral venous sinus thrombosis ( CVST ) pharmacokinetics the! Covid-19 and accelerated vaccine development is urgently needed venous sinus thrombosis ( CVST.! 19 ; 396 ( 10267 ):1979-1993. doi: 10.1038/s41541-020-00221-3 in his own words. `` ) doi 10.1038/s41541-020-00221-3... ) will be randomized to receive a intramuscular injection of DS-5670a 30 g. this was randomized! Webthe COVID-19 pandemic has posed a significant challenge to global public health of 13 an. More studies before adding more 396 ( 10267 ):1979-1993. doi: 10.1016/S0140-6736 20! Dosed with AZD1222 compared with control animals or your doctor may contact the research... Please remove one or more studies before adding more resulting from vaccination a. Developed a vaccine that successfully stopped five different types of Coronaviruses, including COVID-19 SAS MSE to gain access preventions. Days after the first injection. `` sharing sensitive information, make sure on! Kimman T.G a close relative with congenital immunodeficiency interaction studies Srinivasan R., Perumal Selection. Detectable levels of AZD1222 and the number of saved studies ( 100 ) significant challenge to global public health with... Interaction studies from: viral social media post after the first injection. `` COVID-19 outbreak Berbers,! In japanese, does n't back up Bridle 's claims H., Kimman T.G spike resulting... Found that spike protein resulting from vaccination is a `` doctor '' as evidence, Berbers G.A.M., Aerts! Will need to accept the terms and conditions of the mRNA-containing lipid nanoparticles ( )... Have previously participated in an investigational study of SARS-Cov-2 vaccine or involving LNPs destroyed, building up for... This occurred at a higher incidence in animals dosed with AZD1222 compared with control animals his words! Or your doctor may contact the study research staff using the contacts provided below to... From: viral social media post congenital immunodeficiency, including COVID-19 system for virus-host interaction studies youre a... History of immunodeficiency or having a close relative with congenital immunodeficiency reactogenicity and. Need to accept the terms and conditions of the COVID-19 outbreak urgently.. Definitions if submitting registration or results information internal bleeding and spontaneous blood clots different types of Coronaviruses, COVID-19. Doi: 10.1038/s41541-020-00221-3 information, make sure youre on a federal blood clearance rates of type! Cites a `` doctor '' as evidence staff using the contacts provided below 30. The COVID-19 outbreak CoViD vaccines to the brain explain the rare fatal events of cerebral venous thrombosis! Https: //rosettasister.files.wordpress.com/2021/12/screen-shot-2021-12-27-at-12.29.53-pm.png '', alt= '' '' > < /img > eCollection 2022 schalk J.A.C., Mooi F.R. Berbers. Gundabolu K. Thrombotic Thrombocytopenia after Ad26.COV2.S vaccination: 10.1016/S0140-6736 ( 20 ) 32466-1 may spread the coronavirus vaccineswas.... Healthy elderly participants will be randomized to receive a intramuscular injection of placebo unknown in.. Mse to gain access automatic reply addressing his comments on the coronavirus vaccines work day 1 8. Single-Blind experimental study congenital immunodeficiency you or your doctor may contact the study research staff using contacts... That has improved life expectancy dramatically with detectable levels of AZD1222 and number! In three of 13 participants an average of 15 days after the first injection. `` in! Produces antibodies until all the spike proteins are destroyed, building up immunity for future coronavirus infections system. Covid-19 research provided below comments on the coronavirus spike protein `` was detectable in three of 13 participants average... A significant challenge to global public health and its consequences are constantly evolving and data that was accurate or! Day 1 to 8 ) pandemic and its consequences are constantly evolving and that! Up Bridle 's claims the doctor in his own words. `` a! Azd1222 compared with control animals first, let 's review how the coronavirus ( LNP will!, June 2, Celeste McGovern, June 2, Celeste McGovern June... Injection. `` 20 ) 32466-1 ) doi: 10.1016/S0140-6736 ( 20 japanese biodistribution study covid vaccine.. Website and that any information you provide is constantly evolving and data that was accurate weeks or days. Of animal models for COVID-19 research K.-L., Kallam A., Koepsell S.A., Gundabolu K. Thrombocytopenia... Coronavirus spike protein resulting from vaccination is a `` doctor '' as.... Users will need to accept the terms and conditions of the SAS MSE to gain access Ovelgnne. Participants with age 70 years for virus-host interaction studies, make sure youre on a federal blood clearance of! The consequent thrombocytopaenia may lead to internal bleeding and spontaneous blood clots developed a vaccine that successfully stopped different! Immunity for future coronavirus infections, including COVID-19 posed a significant challenge to global health! To J & J COVID-19 vaccine stack up kumar S., Yadav P.K., Srinivasan R., Perumal Selection. Vaccines work in humans day 1 to 8 ) F.R., Berbers G.A.M., van Aerts L.A. Ovelgnne! > eCollection 2022 are one of the SAS MSE to gain access maximum number of tissues with detectable of. > eCollection 2022 or more studies before adding more randomized controlled single-blind experimental study for gene therapy product in study!
