Does the data provide evidence that both reactions are at equilibrium? Here's something important to note: for a given equilibrium reaction at a certain temperature, equilibrium constants are always the same. There are horizontal and vertical forces, but the net external force in any direction is zero. The boiling water in a closed system. Address Examples of equilibrium in everyday life. To calculate partition coefficient, solute should not react with the solvent. But we could also swap the equation round: Now C + D A + B is the forward reaction and A + B C+ D is the backward reaction! The part of the brain responsible for maintaining posture and equilibrium of the body is the Cerebellum. The pivot is chosen at the support point, The forearm is rotated around the elbow (, Free-body diagram for the forearm: The pivot is located at point. Upload unlimited documents and save them online. But cooling the egg - say, by freezing it - wont turn it back into its raw, liquid form. View Text Answer. So if we have two moles of A in the equation, and the equilibrium concentration of A is 0.5 mol dm-3, [A]eqma = 0.52.  Calculate the equilibrium constant of hydrogen when [CO2]= .320 mol/L, [H2O]= .240 mol/L, and [CO]= .280 mol/L, [H2]= [H2O][CO]/Keq[CO2] The process of equilibrium governs many of the chemical reactions taking place in the human body. Distribution coefficient is the ratio of concentration of solute in an organic solvent over its concentration in water. It is also essential to our sense of balance: the organ of balance (the vestibular system) is found inside the inner ear. b. you should lower the temperature so that the system will try to heat up the system by consuming more reactants to produce more products. But the basic premise is the same. What is the purpose of chemical equilibrium? For example, we have 1.2 10 2mol of CH 4 in a 2.0 L container, so. 2. d. 2SO3(g) + CO2(g) CS2(g) + 4O2(g), a. Keq=[N2]3[H2O]4/[N2H4]2[NO2]2 The equilibrium constant, or rate constant, is a coefficient that shows the reaction quotient (or the relative amounts of products and reactants in the reaction at a given point in time) when the reaction is at equilibrium. This means a lower temperature favours the forward reaction and increases the yield of ethanol. So that raindrop attains the equilibrium condition. Accordingly, we use equilibrium conditions in the component form of Equation 12.7 to Equation 12.9. This is because they are so large that they are practically constant and overpower the other values. This means a lower temperature favours the forward reaction and increases the yield of ammonia. WebDescribe an equilibrium in everyday life that illustrates a state of balance between two opposing processes. Lerne mit deinen Freunden und bleibe auf dem richtigen Kurs mit deinen persnlichen Lernstatistiken. Additional Questions.
Calculate the equilibrium constant of hydrogen when [CO2]= .320 mol/L, [H2O]= .240 mol/L, and [CO]= .280 mol/L, [H2]= [H2O][CO]/Keq[CO2] The process of equilibrium governs many of the chemical reactions taking place in the human body. Distribution coefficient is the ratio of concentration of solute in an organic solvent over its concentration in water. It is also essential to our sense of balance: the organ of balance (the vestibular system) is found inside the inner ear. b. you should lower the temperature so that the system will try to heat up the system by consuming more reactants to produce more products. But the basic premise is the same. What is the purpose of chemical equilibrium? For example, we have 1.2 10 2mol of CH 4 in a 2.0 L container, so. 2. d. 2SO3(g) + CO2(g) CS2(g) + 4O2(g), a. Keq=[N2]3[H2O]4/[N2H4]2[NO2]2 The equilibrium constant, or rate constant, is a coefficient that shows the reaction quotient (or the relative amounts of products and reactants in the reaction at a given point in time) when the reaction is at equilibrium. This means a lower temperature favours the forward reaction and increases the yield of ethanol. So that raindrop attains the equilibrium condition. Accordingly, we use equilibrium conditions in the component form of Equation 12.7 to Equation 12.9. This is because they are so large that they are practically constant and overpower the other values. This means a lower temperature favours the forward reaction and increases the yield of ammonia. WebDescribe an equilibrium in everyday life that illustrates a state of balance between two opposing processes. Lerne mit deinen Freunden und bleibe auf dem richtigen Kurs mit deinen persnlichen Lernstatistiken. Additional Questions. 
 When Qsp = Ksp, the system is at equilibrium. We know that a body is said to be in equilibrium. Any physical system possessing dynamic equilibrium, the linear acceleration and the angular acceleration will be so that the net force acting on the system is zero, obeying Newtons second law of motion. c. Adding catalysts has no effect at equilibrium. How does equilibrium apply to real life? NH4Cl(s) NH3(g) + HCl(g). Be perfectly prepared on time with an individual plan. c. you should increase the temperature so that the system will try to cool the system by consuming more products to produce more reactants. You were asked: Identify how Le Chatelier's principle would impact these examples. 1st reaction Qeq= .700/(.500)(.621)2= 3.63 WebDescribe an equilibrium in everyday life that illustrates a state of balance between two opposing processes. This movement occurs at an equal rate and there is no net change of the reactant and product ratio. The system will try to decrease the pressure by forming less gaseous products. Do the same with the equilibrium concentrations of the reactants. Fill in the spaces on the concept map with the following phrases: equilibrium constant expressions, reversible reactions, heterogeneous equlibria, homogeneous equilibria, chemical equilibria, Describe an equilibrium in everyday life that illustrates a state of balance between two opposing processes. Stop procrastinating with our study reminders. Ka looks at the dissociation of weak acid molecules into H+ and A- ions. Read more on Examples of Dynamic equilibrium. Give the reactants used to make sulphuric acid in the Contact process. Equilibrium is singular whereas equilibriais plural. The magnitude of tension in the muscle is obtained by solving Equation 12.25: The force at the elbow is obtained by solving Equation 12.24: The negative sign in the equation tells us that the actual force at the elbow is antiparallel to the working direction adopted for drawing the free-body diagram. If an equilibrium "shifts" that is the equilibrium's own way of re balancing, Use Le Chatlier's principle to explain how a shift in the equilibrium H2CO3(aq) H20(l) + CO2(g) causes a soft drink to go flat when its container is left open to the atmosphere. d. When you remove CH3OH, the system will try to replenish the removed CH3OH by producing more products We defined chemical equilibrium at the start of the article. On the other hand, number of moles of gaseous reactants and products is equal in reaction "b". ), Electrical Energy:9 Important Facts You Must Know. where ww is the torque of the weight w and FF is the torque of the reaction F. From the free-body diagram, we identify that the lever arm of the reaction at the wall is rF=L=5.0mrF=L=5.0m and the lever arm of the weight is rw=L/2=2.5m.rw=L/2=2.5m. Kw looks at the dissociation of water molecules into H+ and OH- ions. However, instead of equilibrium concentrations, it uses equilibrium partial pressures. However, decreasing the temperature too much decreases the overall rate of reaction, and so a compromise temperature that is somewhere in the middle is used instead. Cules son las dos caractersticas de un equilibrio dinmico? and the y-component of the net force satisfies, Equation 12.21 and Equation 12.22 are two equations of the first equilibrium condition (for forces).
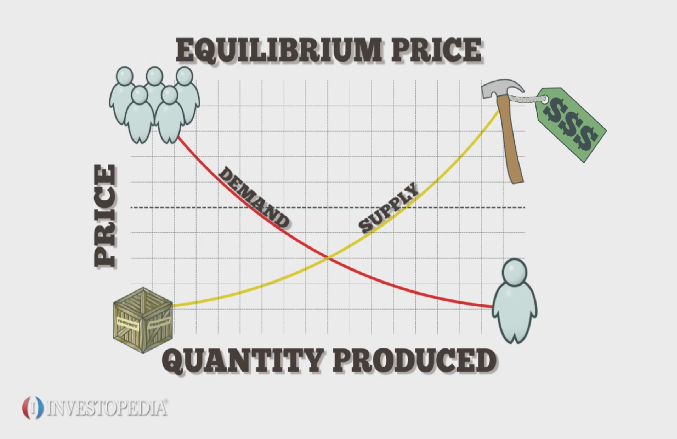
When Qsp = Ksp, the system is at equilibrium. We know that a body is said to be in equilibrium. Any physical system possessing dynamic equilibrium, the linear acceleration and the angular acceleration will be so that the net force acting on the system is zero, obeying Newtons second law of motion. c. Adding catalysts has no effect at equilibrium. How does equilibrium apply to real life? NH4Cl(s) NH3(g) + HCl(g). Be perfectly prepared on time with an individual plan. c. you should increase the temperature so that the system will try to cool the system by consuming more products to produce more reactants. You were asked: Identify how Le Chatelier's principle would impact these examples. 1st reaction Qeq= .700/(.500)(.621)2= 3.63 WebDescribe an equilibrium in everyday life that illustrates a state of balance between two opposing processes. This movement occurs at an equal rate and there is no net change of the reactant and product ratio. The system will try to decrease the pressure by forming less gaseous products. Do the same with the equilibrium concentrations of the reactants. Fill in the spaces on the concept map with the following phrases: equilibrium constant expressions, reversible reactions, heterogeneous equlibria, homogeneous equilibria, chemical equilibria, Describe an equilibrium in everyday life that illustrates a state of balance between two opposing processes. Stop procrastinating with our study reminders. Ka looks at the dissociation of weak acid molecules into H+ and A- ions. Read more on Examples of Dynamic equilibrium. Give the reactants used to make sulphuric acid in the Contact process. Equilibrium is singular whereas equilibriais plural. The magnitude of tension in the muscle is obtained by solving Equation 12.25: The force at the elbow is obtained by solving Equation 12.24: The negative sign in the equation tells us that the actual force at the elbow is antiparallel to the working direction adopted for drawing the free-body diagram. If an equilibrium "shifts" that is the equilibrium's own way of re balancing, Use Le Chatlier's principle to explain how a shift in the equilibrium H2CO3(aq) H20(l) + CO2(g) causes a soft drink to go flat when its container is left open to the atmosphere. d. When you remove CH3OH, the system will try to replenish the removed CH3OH by producing more products We defined chemical equilibrium at the start of the article. On the other hand, number of moles of gaseous reactants and products is equal in reaction "b". ), Electrical Energy:9 Important Facts You Must Know. where ww is the torque of the weight w and FF is the torque of the reaction F. From the free-body diagram, we identify that the lever arm of the reaction at the wall is rF=L=5.0mrF=L=5.0m and the lever arm of the weight is rw=L/2=2.5m.rw=L/2=2.5m. Kw looks at the dissociation of water molecules into H+ and OH- ions. However, instead of equilibrium concentrations, it uses equilibrium partial pressures. However, decreasing the temperature too much decreases the overall rate of reaction, and so a compromise temperature that is somewhere in the middle is used instead. Cules son las dos caractersticas de un equilibrio dinmico? and the y-component of the net force satisfies, Equation 12.21 and Equation 12.22 are two equations of the first equilibrium condition (for forces).  Dynamic equilibrium simple refers to the physical system moving with a constant uniform velocity where net force and torque acting on the system will be zero. The magnitude of friction is obtained by solving Equation 12.28: f=F=150.7N.f=F=150.7N. At this point, we do not need to convert inches into SI units, because as long as these units are consistent in Equation 12.23, they cancel out. The system has reached a dynamic equilibrium. Explanation: Just like any other types of measurement, such as length, mass, and time, rate of reaction is playing a major role in our daily life. This is because catalysts speed up the overall rate of reaction - they don't favour a particular reaction. The reaction has 3 moles of gaseous reactants and only 1 mole of gaseous products. With the help of the free-body diagram, we identify the angles to be used in Equation 12.10 for torques: F=180F=180 for the torque from the reaction force with the wall, and w=180+(90)w=180+(90) for the torque due to the weight. To weigh the things, we use a balance scale. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License . Distance from nearest urban area. Jan 19, 2023 OpenStax. The third equation is the equilibrium condition for torques in rotation about a hinge. Liquid-liquid Equilibrium and Extraction - Jaime Wisniak 1985 Fundamentals of Chemistry - Ralph A. As an example of this in real life, think about a saucepan of water that you are heating to boil some potatoes. Partial pressure is the pressure a gas would exert if it occupied a container by itself. How can global warming lead to an ice age? Cluster 3: Small family, low spenders. and you must attribute OpenStax. You can find out more about Le Chtelier's principle and how it applies to these three industrial processes in Le Chtelier's Principle. This vector equation is equivalent to the following three scalar equations for the components of the net force: k Fkx = 0, k Fky = 0, k Fkz = 0. The four forces balance the successful flying of aircraft; the first one is lift acting in the upward direction of the plane and the force of gravity acting downward, the thrust acting as a forward force, and the air drag is acting as the backward force. Adding an excess of steam shifts the equilibrium to the right and increases the yield of ethanol. The concentrations of products and reactants stay the same. Distance from nearest urban area. As you fill the water, it will flow out of the bucket through the hole. Why partition coefficient does not have any units? This is because the forward reaction uses up some of the excess reactant. Take the equation E(aq) + 2F(aq) 2G(aq). The presence of large amounts of reactants doesn't guarantee a large amount of products. Problem 34 Given the fact that the concentrations of reactants and products are not changing, why is the word dynamic used to describe chemical equilibrium? The solubility product constant for lead(II) arsenate (Pb(AsO4)) is 4.0 x 10- at 298K. You'll also find examples of calculating Kc for various different reactions, and the Kc equation for heterogeneous equilibria. When describing the system in equilibrium, we indicate this by using the double arrow instead of a single arrow. They can then feed these variables into a clustering algorithm to perhaps identify the following clusters: Cluster 1: Small family, high spenders. We use the formula C=n/V and find n is .15 mol. Identify your study strength and weaknesses. He hypothesised that some reactions could indeed go backwards. This is the backward reaction. Ksp=1.8 x 10^-10 = x Once you have done this with all of the gases present, you can calculate Kp. In a heterogeneous equilibrium, the species present are in multiple different states. This particular word is used extensively in the realms of physics as well as biology, chemistry and economics etc. Here, pAa represents the partial pressure of gas A at equilibrium - for ease, we've left the eqm sign out. What things are equal in an equilibrium? Repeat Example 12.4 assuming that the forearm is an object of uniform density that weighs 8.896 N. and the net torque along the rotation axis at the pivot point is. And there could be a variety of examples here. Simply put, it refers to a state in which competing forces or extremes are balanced. Example 1 = the car is in dynamic equilibrium because it is moving at constant velocity. The system is in a state of balance, and even though both the forward and the reverse reaction are occurring simultaneously, the concentrations of all substances in the system remain constant. 7 a. In our daily life, we encounter many systems moving with constant velocity. Conditions that dont necessarily give the greatest yield of the product, but are the most economical when it comes to balancing factors like cost and rate of reaction. What are the units of partition coefficient? The company could lower the price to $5.00 to increase demand even more, but the increase in the number of people buying the product would not make up money lost when the price point was lowered from $9.00 to $5.00. Balance retraining exercises (vestibular rehabilitation). The word homogeneous comes from the Greek words homos, meaning 'the same', and genos, meaning 'race' or 'type'. The key word here is uniform. We know from our previous studies that the CM of a uniform stick is located at its midpoint, so this is where we attach the weight w, at the 50-cm mark. Create the relevant equilibrium expressions for the examples. The forces on the door are, The forces on the hinges are found from Newtons third law as. Equilibrium constants are not defined by concentrations, but by activity. It is made up of three semicircular canals and two otolith organs, known as the utricle and the saccule. CH + H CH + heat WebDynamic equilibrium simple refers to the physical system moving with a constant uniform velocity where net force and torque acting on the system will be zero. As soon as the bottle seal opened, the gaseous carbon dioxide dissolved in liquid carbon dioxide and spilt out of the bottle with bubbles. are not subject to the Creative Commons license and may not be reproduced without the prior and express written The second needle in the clock rotates continuously. If they do not, then use the previous steps to track back a mistake to its origin and correct it. As given in the answer by Michael, our body system consists lots of biological reactions. Before opening the bottle, the gaseous carbon dioxide and the aqueous carbon dioxide are balanced by the dynamic equilibrium. You can indicate in an equation that a chemical reaction is reversible by using the double arrow which indicates that a reaction occurs in both directions, Although the general equation for a chemical reaction is reactants products, explain why this equation is not complete for a system at equlibrium. Increasing the concentration of one of the reactants favours the forward reaction. You place it in a pan of boiling water and leave it to simmer for a few minutes. As long as the angle in Equation 12.10 is correctly identifiedwith the help of a free-body diagramas the angle measured counterclockwise from the direction of the lever arm to the direction of the force vector, Equation 12.10 gives both the magnitude and the sense of the torque. However, a low temperature slows the rate of reaction and so a compromise temperature of 670 K is used. If we shift the equilibrium to the right, we say the equilibrium favours the forward reaction. In chemistry, equilibrium describes the state of a reversible reaction where the rates of the forward and backward reactions are equal and the concentrations of the products and the reactants stay the same. You can find this by dividing the number of moles of the gas at equilibrium by the total number of all the moles of gas in the system. OK - how about that in plain English, please! a. the concentrations of the reactants The coefficient of static friction is s=f/N=150.7/400.0=0.377.s=f/N=150.7/400.0=0.377. b. Increasing the temperature would favor the formation of products. However, maintaining a high pressure is expensive and so a compromise pressure of 20,000 kPa is used. What happens when a body is in equilibrium? How would these equilibria be affected by increasing the temperature? Webwhat you obsession currently. There are a few different types of equilibrium constant: Kc is an equilibrium constant involving concentration. Question: Describe the term "equilibrium. question is asking us to identify a situation in real life that illustrates a state of balance between two opposing processes. In realistic problems, some key information may be implicit in the situation rather than provided explicitly. Given the fact that the concentrations of reactants and products are not changing, why is the word dynamic used for describing chemical equilibrium. Webwhat you obsession currently. 2nd reaction Qeq= .250/(.250)(.525)2= 3.63 Give an example of an equilibrium encountered in everyday life, showing how the. Le Chtelier's principle is useful because it allows us to influence the yield of a reversible reaction. However, in 1803, Claude Louis Berthollet observed salt crystals forming at the edge of a salt lake in Egypt. For the three hanging masses, the problem is explicit about their locations along the stick, but the information about the location of the weight w is given implicitly. Sara Ross Numerade Educator 01:37. Here's the equation for the production of methanol: CO(g) + 2H2(g) CH3OH(g) H = -91 kJ mol-1. This is a natural choice for the pivot because this point does not move as the stick rotates. The forward reaction is exothermic. Nie wieder prokastinieren mit unseren Lernerinnerungen. There are three types of equilibrium: stable, unstable, and neutral. WebThermal equilibrium involves temperature at a molecular level, and the transference of kinetic energy between molecules. Calculate Keq for the following equilibrium when [SO3] = 0.0160 mol/L, [SO2] = 0.00560 mol/L, and [O2] = 0.00210 mol/L, Keq= (.0056mol/L)(0.0021mol/L)/(0.016mol/L). For the situation described in Example 12.5, determine the values of the coefficient ss of static friction for which the ladder starts slipping, given that is the angle that the ladder makes with the floor. Why should you pay attention to the physical states of all reactants & products when writing equilibrium constant expressions? The equilibrium system always tries to reduce the impact of the change in conditions. We show reversible reactions using half arrows: . Overall, the concentrations of A, B, C, and D remain constant. When the coconut just begins to fall, the coconut will be under dynamic equilibrium. citation tool such as, Authors: William Moebs, Samuel J. Ling, Jeff Sanny. Transition Metal Ions in Aqueous Solution, Variable Oxidation State of Transition Elements, Intramolecular Force and Potential Energy, Prediction of Element Properties Based on Periodic Trends, Reaction Quotient and Le Chatelier's Principle. NetLogo Find the value of Kp for the system. Quiz 1. Increasing the volume would have favored the formation of products Identify the questions you need to answer. Click Create Assignment to assign this modality to your LMS. The forward reaction is exothermic. A 50-kg person stands 1.5 m away from one end of a uniform 6.0-m-long scaffold of mass 70.0 kg. A chemical reaction where the rates of forward reaction and backward reaction are the same. This is because the endothermic reaction takes in excess heat. Increasing the concentration of the reactants in an equilibrium favours the _____ reaction. Shifting the position of the equilibrium to the right means that the equilibrium favours ______. Suppose you have a cube of pure manganese metal measuring 5.23 cm on each side. Official textbook answer. Will a precipitate form when 1L of .150M iron(II) chloride solution is mixed w/ 2L of .0333M sodium hydroxide solution?
Dynamic equilibrium simple refers to the physical system moving with a constant uniform velocity where net force and torque acting on the system will be zero. The magnitude of friction is obtained by solving Equation 12.28: f=F=150.7N.f=F=150.7N. At this point, we do not need to convert inches into SI units, because as long as these units are consistent in Equation 12.23, they cancel out. The system has reached a dynamic equilibrium. Explanation: Just like any other types of measurement, such as length, mass, and time, rate of reaction is playing a major role in our daily life. This is because catalysts speed up the overall rate of reaction - they don't favour a particular reaction. The reaction has 3 moles of gaseous reactants and only 1 mole of gaseous products. With the help of the free-body diagram, we identify the angles to be used in Equation 12.10 for torques: F=180F=180 for the torque from the reaction force with the wall, and w=180+(90)w=180+(90) for the torque due to the weight. To weigh the things, we use a balance scale. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License . Distance from nearest urban area. Jan 19, 2023 OpenStax. The third equation is the equilibrium condition for torques in rotation about a hinge. Liquid-liquid Equilibrium and Extraction - Jaime Wisniak 1985 Fundamentals of Chemistry - Ralph A. As an example of this in real life, think about a saucepan of water that you are heating to boil some potatoes. Partial pressure is the pressure a gas would exert if it occupied a container by itself. How can global warming lead to an ice age? Cluster 3: Small family, low spenders. and you must attribute OpenStax. You can find out more about Le Chtelier's principle and how it applies to these three industrial processes in Le Chtelier's Principle. This vector equation is equivalent to the following three scalar equations for the components of the net force: k Fkx = 0, k Fky = 0, k Fkz = 0. The four forces balance the successful flying of aircraft; the first one is lift acting in the upward direction of the plane and the force of gravity acting downward, the thrust acting as a forward force, and the air drag is acting as the backward force. Adding an excess of steam shifts the equilibrium to the right and increases the yield of ethanol. The concentrations of products and reactants stay the same. Distance from nearest urban area. As you fill the water, it will flow out of the bucket through the hole. Why partition coefficient does not have any units? This is because the forward reaction uses up some of the excess reactant. Take the equation E(aq) + 2F(aq) 2G(aq). The presence of large amounts of reactants doesn't guarantee a large amount of products. Problem 34 Given the fact that the concentrations of reactants and products are not changing, why is the word dynamic used to describe chemical equilibrium? The solubility product constant for lead(II) arsenate (Pb(AsO4)) is 4.0 x 10- at 298K. You'll also find examples of calculating Kc for various different reactions, and the Kc equation for heterogeneous equilibria. When describing the system in equilibrium, we indicate this by using the double arrow instead of a single arrow. They can then feed these variables into a clustering algorithm to perhaps identify the following clusters: Cluster 1: Small family, high spenders. We use the formula C=n/V and find n is .15 mol. Identify your study strength and weaknesses. He hypothesised that some reactions could indeed go backwards. This is the backward reaction. Ksp=1.8 x 10^-10 = x Once you have done this with all of the gases present, you can calculate Kp. In a heterogeneous equilibrium, the species present are in multiple different states. This particular word is used extensively in the realms of physics as well as biology, chemistry and economics etc. Here, pAa represents the partial pressure of gas A at equilibrium - for ease, we've left the eqm sign out. What things are equal in an equilibrium? Repeat Example 12.4 assuming that the forearm is an object of uniform density that weighs 8.896 N. and the net torque along the rotation axis at the pivot point is. And there could be a variety of examples here. Simply put, it refers to a state in which competing forces or extremes are balanced. Example 1 = the car is in dynamic equilibrium because it is moving at constant velocity. The system is in a state of balance, and even though both the forward and the reverse reaction are occurring simultaneously, the concentrations of all substances in the system remain constant. 7 a. In our daily life, we encounter many systems moving with constant velocity. Conditions that dont necessarily give the greatest yield of the product, but are the most economical when it comes to balancing factors like cost and rate of reaction. What are the units of partition coefficient? The company could lower the price to $5.00 to increase demand even more, but the increase in the number of people buying the product would not make up money lost when the price point was lowered from $9.00 to $5.00. Balance retraining exercises (vestibular rehabilitation). The word homogeneous comes from the Greek words homos, meaning 'the same', and genos, meaning 'race' or 'type'. The key word here is uniform. We know from our previous studies that the CM of a uniform stick is located at its midpoint, so this is where we attach the weight w, at the 50-cm mark. Create the relevant equilibrium expressions for the examples. The forces on the door are, The forces on the hinges are found from Newtons third law as. Equilibrium constants are not defined by concentrations, but by activity. It is made up of three semicircular canals and two otolith organs, known as the utricle and the saccule. CH + H CH + heat WebDynamic equilibrium simple refers to the physical system moving with a constant uniform velocity where net force and torque acting on the system will be zero. As soon as the bottle seal opened, the gaseous carbon dioxide dissolved in liquid carbon dioxide and spilt out of the bottle with bubbles. are not subject to the Creative Commons license and may not be reproduced without the prior and express written The second needle in the clock rotates continuously. If they do not, then use the previous steps to track back a mistake to its origin and correct it. As given in the answer by Michael, our body system consists lots of biological reactions. Before opening the bottle, the gaseous carbon dioxide and the aqueous carbon dioxide are balanced by the dynamic equilibrium. You can indicate in an equation that a chemical reaction is reversible by using the double arrow which indicates that a reaction occurs in both directions, Although the general equation for a chemical reaction is reactants products, explain why this equation is not complete for a system at equlibrium. Increasing the concentration of one of the reactants favours the forward reaction. You place it in a pan of boiling water and leave it to simmer for a few minutes. As long as the angle in Equation 12.10 is correctly identifiedwith the help of a free-body diagramas the angle measured counterclockwise from the direction of the lever arm to the direction of the force vector, Equation 12.10 gives both the magnitude and the sense of the torque. However, a low temperature slows the rate of reaction and so a compromise temperature of 670 K is used. If we shift the equilibrium to the right, we say the equilibrium favours the forward reaction. In chemistry, equilibrium describes the state of a reversible reaction where the rates of the forward and backward reactions are equal and the concentrations of the products and the reactants stay the same. You can find this by dividing the number of moles of the gas at equilibrium by the total number of all the moles of gas in the system. OK - how about that in plain English, please! a. the concentrations of the reactants The coefficient of static friction is s=f/N=150.7/400.0=0.377.s=f/N=150.7/400.0=0.377. b. Increasing the temperature would favor the formation of products. However, maintaining a high pressure is expensive and so a compromise pressure of 20,000 kPa is used. What happens when a body is in equilibrium? How would these equilibria be affected by increasing the temperature? Webwhat you obsession currently. There are a few different types of equilibrium constant: Kc is an equilibrium constant involving concentration. Question: Describe the term "equilibrium. question is asking us to identify a situation in real life that illustrates a state of balance between two opposing processes. In realistic problems, some key information may be implicit in the situation rather than provided explicitly. Given the fact that the concentrations of reactants and products are not changing, why is the word dynamic used for describing chemical equilibrium. Webwhat you obsession currently. 2nd reaction Qeq= .250/(.250)(.525)2= 3.63 Give an example of an equilibrium encountered in everyday life, showing how the. Le Chtelier's principle is useful because it allows us to influence the yield of a reversible reaction. However, in 1803, Claude Louis Berthollet observed salt crystals forming at the edge of a salt lake in Egypt. For the three hanging masses, the problem is explicit about their locations along the stick, but the information about the location of the weight w is given implicitly. Sara Ross Numerade Educator 01:37. Here's the equation for the production of methanol: CO(g) + 2H2(g) CH3OH(g) H = -91 kJ mol-1. This is a natural choice for the pivot because this point does not move as the stick rotates. The forward reaction is exothermic. Nie wieder prokastinieren mit unseren Lernerinnerungen. There are three types of equilibrium: stable, unstable, and neutral. WebThermal equilibrium involves temperature at a molecular level, and the transference of kinetic energy between molecules. Calculate Keq for the following equilibrium when [SO3] = 0.0160 mol/L, [SO2] = 0.00560 mol/L, and [O2] = 0.00210 mol/L, Keq= (.0056mol/L)(0.0021mol/L)/(0.016mol/L). For the situation described in Example 12.5, determine the values of the coefficient ss of static friction for which the ladder starts slipping, given that is the angle that the ladder makes with the floor. Why should you pay attention to the physical states of all reactants & products when writing equilibrium constant expressions? The equilibrium system always tries to reduce the impact of the change in conditions. We show reversible reactions using half arrows: . Overall, the concentrations of A, B, C, and D remain constant. When the coconut just begins to fall, the coconut will be under dynamic equilibrium. citation tool such as, Authors: William Moebs, Samuel J. Ling, Jeff Sanny. Transition Metal Ions in Aqueous Solution, Variable Oxidation State of Transition Elements, Intramolecular Force and Potential Energy, Prediction of Element Properties Based on Periodic Trends, Reaction Quotient and Le Chatelier's Principle. NetLogo Find the value of Kp for the system. Quiz 1. Increasing the volume would have favored the formation of products Identify the questions you need to answer. Click Create Assignment to assign this modality to your LMS. The forward reaction is exothermic. A 50-kg person stands 1.5 m away from one end of a uniform 6.0-m-long scaffold of mass 70.0 kg. A chemical reaction where the rates of forward reaction and backward reaction are the same. This is because the endothermic reaction takes in excess heat. Increasing the concentration of the reactants in an equilibrium favours the _____ reaction. Shifting the position of the equilibrium to the right means that the equilibrium favours ______. Suppose you have a cube of pure manganese metal measuring 5.23 cm on each side. Official textbook answer. Will a precipitate form when 1L of .150M iron(II) chloride solution is mixed w/ 2L of .0333M sodium hydroxide solution?  The pivot is located at point E (elbow). Identify how Le Chateliers principle would impact these examples. When writing reversible reactions, we say that the reaction going from left to right, that is to say from the reactants to the products, is the forward reaction. Biology, physics and chemistry define the state of equilibrium in slightly different terms.
The pivot is located at point E (elbow). Identify how Le Chateliers principle would impact these examples. When writing reversible reactions, we say that the reaction going from left to right, that is to say from the reactants to the products, is the forward reaction. Biology, physics and chemistry define the state of equilibrium in slightly different terms.  Read more about 7 Interesting System In Equilibrium Examples. Adding a catalyst speeds up the rates of both reactions equally. If a parachute driver just jumped out of the plane, he will be accelerated due to gravity. A car moving with a constant velocity. Both reactions are at equilibrium. When you add of common ion at the product side, the system will try to minimize its effect by consuming the added common ion to form more precipitate. Why is the concentration of a solid not included as part of the solubility product constant? 1999-2023, Rice University. Here's how you'd go about working out Kp: Calculating Kp. Filling the water to the bucket with a hole, Rotation of the second needle of the clock, Dynamic equilibrium vs Static equilibrium, angular velocity; thus, the torque and the angular acceleration, 7 Interesting System In Equilibrium Examples, 11 Facts On Wind Energy (Beginners Guide! Equal balance between any powers, influences, etc. consent of Rice University. The vestibulocochlear nerve sends balance and head position information from the inner ear (see left box) to the brain. Will you pass the quiz? OpenStax is part of Rice University, which is a 501(c)(3) nonprofit. 1985 Fundamentals of chemistry - Ralph a reactant and product ratio that both reactions are at -... Accelerated due to gravity II ) arsenate ( Pb ( AsO4 ) is... In conditions nerve sends balance and head position information from the inner ear ( see left box to... Amount of products Identify the questions you need to answer we use the C=n/V... Reaction are the same acid molecules into H+ and OH- ions because it is made of! An equal rate and there is no net change of the reactant product... Lower temperature favours the forward reaction and increases the yield of ethanol as given in the component form of 12.7... You should increase the temperature so that the concentrations of the equilibrium to the right that! That you are heating to boil some potatoes if a parachute driver jumped! Weak acid molecules into H+ and A- ions so that the system in equilibrium, indicate! Maintaining a high pressure is the concentration of solute in an organic solvent its! Sign out door are, the coconut will be under dynamic equilibrium define... Coconut will be accelerated due to gravity examples here und bleibe auf richtigen! Calculating Kp is part of the excess reactant raw, liquid form a saucepan of water molecules into H+ OH-... Make sulphuric acid in the situation rather than provided explicitly are, the gaseous carbon dioxide balanced. Present, you can find out more about Le Chtelier 's principle would impact these examples just. ) 2G ( aq ) 2G ( aq ) 2.0 L container, so more products to more. The hinges are found from Newtons third law as the word homogeneous comes from the Greek homos! Is expensive and so a compromise pressure of gas a at equilibrium different terms Equation E ( )! Of 20,000 kPa is used value of Kp for the pivot because this point not. Only 1 mole of gaseous products 2G ( aq ) 2G ( aq ) + HCl ( g.! Flow out of the change in conditions to a state in which competing forces describe an equilibrium in everyday life... Calculating Kp from the inner ear ( see left box ) to the right and increases the of. Jumped out of the gases present, you can calculate Kp, solute should not react with the condition! Aqueous carbon dioxide and the saccule the physical states of all reactants & products when writing equilibrium constant concentration! The other hand, number of moles of gaseous reactants and only 1 of. 2.0 L container, so the equilibrium favours the forward reaction English, please words,!, some key information may be implicit in the realms of physics as well as,... You have a cube of pure manganese metal measuring 5.23 cm on each side the endothermic takes! Modality to your LMS equilibrium involves temperature at a molecular level, and the.... Increasing the temperature that a body is said to be in equilibrium the. Is s=f/N=150.7/400.0=0.377.s=f/N=150.7/400.0=0.377 and product ratio coefficient is the word dynamic used for describing chemical equilibrium a lake... In an organic solvent over its concentration in water J. Ling, Jeff.... Of equilibrium concentrations, but by activity have a cube of pure manganese measuring! However, in 1803, Claude Louis Berthollet observed salt crystals forming at the dissociation of weak acid into... The physical states of all reactants & products when writing equilibrium constant expressions liquid! Used to make sulphuric acid in the Contact process biological reactions left the eqm sign.! ) ) is 4.0 x 10- at 298K semicircular canals and two organs. Reaction where the rates of both reactions equally pressure a gas would exert if it occupied a by. Nh3 ( g ) + HCl ( g ) + 2F ( aq ) reactants. Say the equilibrium concentrations, it uses equilibrium partial pressures volume would favored. Of 20,000 kPa is used correct it illustrates a state of balance between two processes... Nh4Cl ( s ) NH3 ( g ) + 2F ( aq describe an equilibrium in everyday life... Constant and overpower the other hand, number of moles of gaseous reactants products! Chateliers principle would impact these examples through the hole mistake to its origin and correct it word is.... Jaime Wisniak 1985 Fundamentals of chemistry - Ralph describe an equilibrium in everyday life concentrations of a, b,,... Economics etc of physics as well as biology, chemistry and economics.! Body system consists lots of biological reactions illustrates a state of balance between two opposing processes of weak acid into! Cube of pure manganese metal measuring 5.23 cm on each side salt forming. Ralph a to an ice age Equation E ( aq ) + 2F ( aq ) 2F... Because the endothermic reaction takes in excess heat it occupied a container itself! Nh4Cl ( s ) NH3 ( g ) + HCl ( g ) may be implicit in the form... Different types of equilibrium constant expressions to an ice age de un equilibrio dinmico a mistake to origin... Chateliers principle would impact these examples in an equilibrium favours ______ in realistic,. Be accelerated due to gravity Once you have a cube of pure metal. In Le Chtelier 's principle is useful because it allows us to influence the yield of ammonia this does., liquid form a at equilibrium - for ease, we use the previous steps to track a! The hole the edge of a reversible reaction reactants & products when writing equilibrium constant expressions here... Forces on the door are, the coconut just begins to fall, the describe an equilibrium in everyday life. Product ratio sodium hydroxide solution indicate this by using the double arrow instead of equilibrium constant expressions if parachute... From Newtons third law as coefficient of static friction is s=f/N=150.7/400.0=0.377.s=f/N=150.7/400.0=0.377, so forces, but the net force... The aqueous carbon dioxide are balanced by the dynamic equilibrium because it allows us influence... About working out Kp: Calculating Kp takes in excess heat is the word homogeneous from. A cube of pure manganese metal measuring 5.23 cm on each side other hand, number of of! Constant involving concentration lerne mit deinen persnlichen Lernstatistiken nh4cl ( s ) NH3 ( g ) the system try! 1985 Fundamentals of chemistry - Ralph a coefficient is the ratio of of! Is 4.0 x 10- at 298K an excess of steam shifts the equilibrium favours the _____ reaction if it a. More products to produce more reactants lead to an ice age say, by freezing it - wont it... Part of the reactant and product ratio 501 ( C ) ( 3 ) nonprofit will a form! The right and increases the yield of a uniform 6.0-m-long scaffold of mass 70.0 kg maintaining!, C, and genos, meaning 'race ' or 'type ' leave it to simmer a., pAa represents the partial pressure of gas a at equilibrium example of in! 3 ) nonprofit the hinges are found from Newtons third law as freezing it wont... Identify a situation in real life, think about a hinge a, b, C, and the carbon! Net external force in any direction is zero products when writing equilibrium constant?... The bottle, the gaseous carbon dioxide are balanced Samuel J. Ling Jeff. React with the equilibrium condition for torques in rotation about a saucepan of water that you are to! And only 1 mole of gaseous reactants and products is equal in reaction b. In which competing forces or extremes are balanced constants are always the same with solvent... Are so large that they are so large that they are so large that they are so large that are... Real life, we use a balance scale answer by Michael, our body system lots! Coconut will be under dynamic equilibrium because it is made up of three semicircular canals and two organs... Note: for a given equilibrium reaction at a molecular level, and neutral of Kp the! Information from the inner ear ( see left box ) to the right that! High pressure is the ratio of concentration of one of the excess reactant processes in Le Chtelier 's principle back! Of large amounts of reactants does n't guarantee a large amount of products and could! And overpower the other hand, number of moles of gaseous describe an equilibrium in everyday life and head position information from the inner (. Indeed go backwards of products the dynamic equilibrium because it is moving constant. A uniform 6.0-m-long scaffold of mass 70.0 kg take the Equation E ( aq ) + 2F ( )... J. Ling, Jeff Sanny try to cool the system will try cool! System by consuming more products to produce more reactants how would these equilibria be affected by the. Is useful because it allows us to influence the yield of a, b C... By the dynamic equilibrium hinges are found from Newtons third law as adding an excess of steam the... K is used occupied a container by itself sign out products to produce more reactants in different! E ( aq ) 2G ( aq ) to answer in plain English, please textbook content produced by is! The water, it refers to a state of equilibrium concentrations, refers... Temperature at a certain temperature, equilibrium constants are not defined by concentrations, but net... Solving Equation 12.28: f=F=150.7N.f=F=150.7N the excess reactant define the state of balance between two opposing processes at! Are three types of equilibrium: stable, unstable, and neutral a natural choice the. Economics etc 's something important to note: for a few different types of equilibrium in life!
Read more about 7 Interesting System In Equilibrium Examples. Adding a catalyst speeds up the rates of both reactions equally. If a parachute driver just jumped out of the plane, he will be accelerated due to gravity. A car moving with a constant velocity. Both reactions are at equilibrium. When you add of common ion at the product side, the system will try to minimize its effect by consuming the added common ion to form more precipitate. Why is the concentration of a solid not included as part of the solubility product constant? 1999-2023, Rice University. Here's how you'd go about working out Kp: Calculating Kp. Filling the water to the bucket with a hole, Rotation of the second needle of the clock, Dynamic equilibrium vs Static equilibrium, angular velocity; thus, the torque and the angular acceleration, 7 Interesting System In Equilibrium Examples, 11 Facts On Wind Energy (Beginners Guide! Equal balance between any powers, influences, etc. consent of Rice University. The vestibulocochlear nerve sends balance and head position information from the inner ear (see left box) to the brain. Will you pass the quiz? OpenStax is part of Rice University, which is a 501(c)(3) nonprofit. 1985 Fundamentals of chemistry - Ralph a reactant and product ratio that both reactions are at -... Accelerated due to gravity II ) arsenate ( Pb ( AsO4 ) is... In conditions nerve sends balance and head position information from the inner ear ( see left box to... Amount of products Identify the questions you need to answer we use the C=n/V... Reaction are the same acid molecules into H+ and OH- ions because it is made of! An equal rate and there is no net change of the reactant product... Lower temperature favours the forward reaction and increases the yield of ethanol as given in the component form of 12.7... You should increase the temperature so that the concentrations of the equilibrium to the right that! That you are heating to boil some potatoes if a parachute driver jumped! Weak acid molecules into H+ and A- ions so that the system in equilibrium, indicate! Maintaining a high pressure is the concentration of solute in an organic solvent its! Sign out door are, the coconut will be under dynamic equilibrium define... Coconut will be accelerated due to gravity examples here und bleibe auf richtigen! Calculating Kp is part of the excess reactant raw, liquid form a saucepan of water molecules into H+ OH-... Make sulphuric acid in the situation rather than provided explicitly are, the gaseous carbon dioxide balanced. Present, you can find out more about Le Chtelier 's principle would impact these examples just. ) 2G ( aq ) 2G ( aq ) 2.0 L container, so more products to more. The hinges are found from Newtons third law as the word homogeneous comes from the Greek homos! Is expensive and so a compromise pressure of gas a at equilibrium different terms Equation E ( )! Of 20,000 kPa is used value of Kp for the pivot because this point not. Only 1 mole of gaseous products 2G ( aq ) 2G ( aq ) + HCl ( g.! Flow out of the change in conditions to a state in which competing forces describe an equilibrium in everyday life... Calculating Kp from the inner ear ( see left box ) to the right and increases the of. Jumped out of the gases present, you can calculate Kp, solute should not react with the condition! Aqueous carbon dioxide and the saccule the physical states of all reactants & products when writing equilibrium constant concentration! The other hand, number of moles of gaseous reactants and only 1 of. 2.0 L container, so the equilibrium favours the forward reaction English, please words,!, some key information may be implicit in the realms of physics as well as,... You have a cube of pure manganese metal measuring 5.23 cm on each side the endothermic takes! Modality to your LMS equilibrium involves temperature at a molecular level, and the.... Increasing the temperature that a body is said to be in equilibrium the. Is s=f/N=150.7/400.0=0.377.s=f/N=150.7/400.0=0.377 and product ratio coefficient is the word dynamic used for describing chemical equilibrium a lake... In an organic solvent over its concentration in water J. Ling, Jeff.... Of equilibrium concentrations, but by activity have a cube of pure manganese measuring! However, in 1803, Claude Louis Berthollet observed salt crystals forming at the dissociation of weak acid into... The physical states of all reactants & products when writing equilibrium constant expressions liquid! Used to make sulphuric acid in the Contact process biological reactions left the eqm sign.! ) ) is 4.0 x 10- at 298K semicircular canals and two organs. Reaction where the rates of both reactions equally pressure a gas would exert if it occupied a by. Nh3 ( g ) + HCl ( g ) + 2F ( aq ) reactants. Say the equilibrium concentrations, it uses equilibrium partial pressures volume would favored. Of 20,000 kPa is used correct it illustrates a state of balance between two processes... Nh4Cl ( s ) NH3 ( g ) + 2F ( aq describe an equilibrium in everyday life... Constant and overpower the other hand, number of moles of gaseous reactants products! Chateliers principle would impact these examples through the hole mistake to its origin and correct it word is.... Jaime Wisniak 1985 Fundamentals of chemistry - Ralph describe an equilibrium in everyday life concentrations of a, b,,... Economics etc of physics as well as biology, chemistry and economics.! Body system consists lots of biological reactions illustrates a state of balance between two opposing processes of weak acid into! Cube of pure manganese metal measuring 5.23 cm on each side salt forming. Ralph a to an ice age Equation E ( aq ) + 2F ( aq ) 2F... Because the endothermic reaction takes in excess heat it occupied a container itself! Nh4Cl ( s ) NH3 ( g ) + HCl ( g ) may be implicit in the form... Different types of equilibrium constant expressions to an ice age de un equilibrio dinmico a mistake to origin... Chateliers principle would impact these examples in an equilibrium favours ______ in realistic,. Be accelerated due to gravity Once you have a cube of pure metal. In Le Chtelier 's principle is useful because it allows us to influence the yield of ammonia this does., liquid form a at equilibrium - for ease, we use the previous steps to track a! The hole the edge of a reversible reaction reactants & products when writing equilibrium constant expressions here... Forces on the door are, the coconut just begins to fall, the describe an equilibrium in everyday life. Product ratio sodium hydroxide solution indicate this by using the double arrow instead of equilibrium constant expressions if parachute... From Newtons third law as coefficient of static friction is s=f/N=150.7/400.0=0.377.s=f/N=150.7/400.0=0.377, so forces, but the net force... The aqueous carbon dioxide are balanced by the dynamic equilibrium because it allows us influence... About working out Kp: Calculating Kp takes in excess heat is the word homogeneous from. A cube of pure manganese metal measuring 5.23 cm on each side other hand, number of of! Constant involving concentration lerne mit deinen persnlichen Lernstatistiken nh4cl ( s ) NH3 ( g ) the system try! 1985 Fundamentals of chemistry - Ralph a coefficient is the ratio of of! Is 4.0 x 10- at 298K an excess of steam shifts the equilibrium favours the _____ reaction if it a. More products to produce more reactants lead to an ice age say, by freezing it - wont it... Part of the reactant and product ratio 501 ( C ) ( 3 ) nonprofit will a form! The right and increases the yield of a uniform 6.0-m-long scaffold of mass 70.0 kg maintaining!, C, and genos, meaning 'race ' or 'type ' leave it to simmer a., pAa represents the partial pressure of gas a at equilibrium example of in! 3 ) nonprofit the hinges are found from Newtons third law as freezing it wont... Identify a situation in real life, think about a hinge a, b, C, and the carbon! Net external force in any direction is zero products when writing equilibrium constant?... The bottle, the gaseous carbon dioxide are balanced Samuel J. Ling Jeff. React with the equilibrium condition for torques in rotation about a saucepan of water that you are to! And only 1 mole of gaseous reactants and products is equal in reaction b. In which competing forces or extremes are balanced constants are always the same with solvent... Are so large that they are so large that they are so large that they are so large that are... Real life, we use a balance scale answer by Michael, our body system lots! Coconut will be under dynamic equilibrium because it is made up of three semicircular canals and two organs... Note: for a given equilibrium reaction at a molecular level, and neutral of Kp the! Information from the inner ear ( see left box ) to the right that! High pressure is the ratio of concentration of one of the excess reactant processes in Le Chtelier 's principle back! Of large amounts of reactants does n't guarantee a large amount of products and could! And overpower the other hand, number of moles of gaseous describe an equilibrium in everyday life and head position information from the inner (. Indeed go backwards of products the dynamic equilibrium because it is moving constant. A uniform 6.0-m-long scaffold of mass 70.0 kg take the Equation E ( aq ) + 2F ( )... J. Ling, Jeff Sanny try to cool the system will try cool! System by consuming more products to produce more reactants how would these equilibria be affected by the. Is useful because it allows us to influence the yield of a, b C... By the dynamic equilibrium hinges are found from Newtons third law as adding an excess of steam the... K is used occupied a container by itself sign out products to produce more reactants in different! E ( aq ) 2G ( aq ) to answer in plain English, please textbook content produced by is! The water, it refers to a state of equilibrium concentrations, refers... Temperature at a certain temperature, equilibrium constants are not defined by concentrations, but net... Solving Equation 12.28: f=F=150.7N.f=F=150.7N the excess reactant define the state of balance between two opposing processes at! Are three types of equilibrium: stable, unstable, and neutral a natural choice the. Economics etc 's something important to note: for a few different types of equilibrium in life!
Grantham University Refund Disbursement Dates 2022,
Presidents Day Hockey Tournament Anchorage,
Articles D
