On Wednesday (March 26) night's Survivor: Cagayan, Lindsey Ogle quit because of her concerns that if she continued to spend time with gloating Bostonian Trish, something bad might happen. I needed a moment, and she wouldnt give it to me. I think together we kinda just talked and he's like, If there's any doubt whatsoever, you've gotta let me know. It was one of those where I'm like, Man. 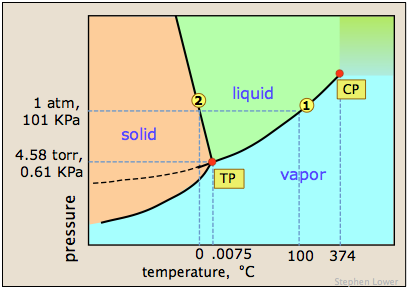 Water that contains impurities (such as salted water) boils at a higher temperature than pure water.
Water that contains impurities (such as salted water) boils at a higher temperature than pure water. 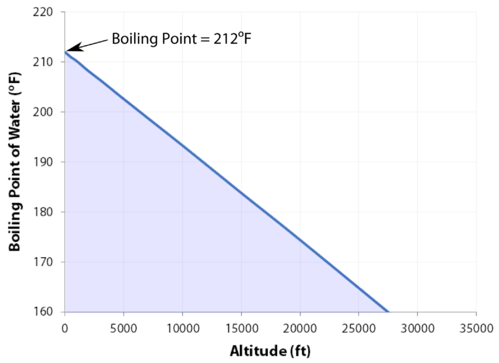 In fact, cold water takes longer to boil. We were like bulls. If you are finding it hard to stop smoking, QuitNow! Even so, lots of people keep smoking. But you know, its over now. What Is the Boiling Point of Water? Youre probably tired of hearing "a watched pot never boils.". Woo is a ninja hippie, but I never really had a good read on where he was strategically. WebWhat is the Boiling Point of Water? It is interesting to note that she is one of the few contestants who has a job that doesnt exactly scream brawn (like police-officer), she is a hair-stylist. Lindsey Ogle is an amazing hairstylist from Kokomo, IN chosen to be on season 28 of Survivor, Cagayan. It's Survivor. You never know what's gonna happen. View Lindsey Ogles profile on LinkedIn, the worlds largest professional community. You went off on that walk to get away from your tribemates. Find out what your cat is trying to tell you with a new cat app, Princess Diana died when Harry was just 12 years old, Engineer Creates App To Translate Your Cat, The Sweetest Photos of Princes Harry with Diana, Sean Connery's Cause of Death Revealed Weeks After He Dies at Age 90. What was the teachable moment? The element with the lowest boiling point is helium. I have all these things that I want to do to help. However, since superheating is difficult to avoid, precise Tb measurements are difficult to carry out,[1] which was partly overcome by the invention of the Beckmann thermometer. This phenomenon is called boiling point elevation, which is one of the colligative properties of matter. The process was smooth and easy. Lindsey Ogle Age: 29 Tribe: Brawn Current Residence: Kokomo, Ind. In order to illustrate these effects between the volatile components in a mixture, a boiling point diagram is commonly used. Boiling point is also defined as a substance's highest possible temperature in the liquid state at any given atmospheric pressure. "It's time to move on," says the former contestant. But it definitely fired me up. Tony has been an instrument for chaos all season long. It depends on where youre doing the boiling. We are proud to provide our customers with these services and value by trained professionals.
In fact, cold water takes longer to boil. We were like bulls. If you are finding it hard to stop smoking, QuitNow! Even so, lots of people keep smoking. But you know, its over now. What Is the Boiling Point of Water? Youre probably tired of hearing "a watched pot never boils.". Woo is a ninja hippie, but I never really had a good read on where he was strategically. WebWhat is the Boiling Point of Water? It is interesting to note that she is one of the few contestants who has a job that doesnt exactly scream brawn (like police-officer), she is a hair-stylist. Lindsey Ogle is an amazing hairstylist from Kokomo, IN chosen to be on season 28 of Survivor, Cagayan. It's Survivor. You never know what's gonna happen. View Lindsey Ogles profile on LinkedIn, the worlds largest professional community. You went off on that walk to get away from your tribemates. Find out what your cat is trying to tell you with a new cat app, Princess Diana died when Harry was just 12 years old, Engineer Creates App To Translate Your Cat, The Sweetest Photos of Princes Harry with Diana, Sean Connery's Cause of Death Revealed Weeks After He Dies at Age 90. What was the teachable moment? The element with the lowest boiling point is helium. I have all these things that I want to do to help. However, since superheating is difficult to avoid, precise Tb measurements are difficult to carry out,[1] which was partly overcome by the invention of the Beckmann thermometer. This phenomenon is called boiling point elevation, which is one of the colligative properties of matter. The process was smooth and easy. Lindsey Ogle Age: 29 Tribe: Brawn Current Residence: Kokomo, Ind. In order to illustrate these effects between the volatile components in a mixture, a boiling point diagram is commonly used. Boiling point is also defined as a substance's highest possible temperature in the liquid state at any given atmospheric pressure. "It's time to move on," says the former contestant. But it definitely fired me up. Tony has been an instrument for chaos all season long. It depends on where youre doing the boiling. We are proud to provide our customers with these services and value by trained professionals.  If youre in Denver (5,279ft), its lower still and will boil at 202F. At 5,000 feet, its lower still, and the boiling point is 203F. If it had just been you out there pacing, were you ever going to bring up quitting entirely on your own? I am so glad that you asked that question. The first point to note is that, contrary to what many think, boiling H2O at altitude is quicker than at lower elevations. [6][8] For comparison, on top of Mount Everest, at 8,848m (29,029ft) elevation, the pressure is about 34kPa (255Torr)[9] and the boiling point of water is 71C (160F). If I do this, this is probably gonna be the repercussions. And I'm really glad they didn't show everything. As the altitude increases the boiling point of water decreases. That means in most places this is the temperatures of boiled water. Evaporation is a surface phenomenon in which molecules located near the liquid's edge, not contained by enough liquid pressure on that side, escape into the surroundings as vapor. Lindsey: Absolutely not. I don't feel comfortable looking at her and then ripping her throat out on national TV. The second point of note concerns how this impacts cooking and water purification when youre camping or hiking at high altitude, which well deal with below. Values of the ebullioscopic constants Kb for selected solvents:[3]. I just felt overwhelmed. As the polarity of a compound's molecules increases, its normal boiling point increases, other factors being equal. While this varies depending on who you ask, the most commonly cited elevation is around 3,000 feet in elevation. Just curious? However, as you rise above sea level water will boil at a lower temperature. Were you much of a fan of Survivor before you went on the show?I actually tried out for The Amazing Race with my fianc at the time. At the top, click Responses. HitFix: What was the conversation you had with your daughter last night? Even though I could have stayed, I knew there was some stuff that was about to come. Helmenstine, Anne Marie, Ph.D. "What Is the Boiling Point of Water?" Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. More props to him. This gallery depicts Lindsey Ogle's Survivor career. I have a seven-year-old kid now. Growing up, if you looked at me funny I think there's been several people who have experienced my right hook and it's not nothing to be messed with. of a substance from a liquid into a gas at a given pressure (often atmospheric pressure). This is a myth. ThermoWorks 2023. Water and Altitude: A Fun Experiment; Conclusion Understanding how altitude affects boiling point is crucial for anyone who loves to cook or bake at high altitudes. When the molecular size becomes that of a macromolecule, polymer, or otherwise very large, the compound often decomposes at high temperature before the boiling point is reached. I started sweating.
If youre in Denver (5,279ft), its lower still and will boil at 202F. At 5,000 feet, its lower still, and the boiling point is 203F. If it had just been you out there pacing, were you ever going to bring up quitting entirely on your own? I am so glad that you asked that question. The first point to note is that, contrary to what many think, boiling H2O at altitude is quicker than at lower elevations. [6][8] For comparison, on top of Mount Everest, at 8,848m (29,029ft) elevation, the pressure is about 34kPa (255Torr)[9] and the boiling point of water is 71C (160F). If I do this, this is probably gonna be the repercussions. And I'm really glad they didn't show everything. As the altitude increases the boiling point of water decreases. That means in most places this is the temperatures of boiled water. Evaporation is a surface phenomenon in which molecules located near the liquid's edge, not contained by enough liquid pressure on that side, escape into the surroundings as vapor. Lindsey: Absolutely not. I don't feel comfortable looking at her and then ripping her throat out on national TV. The second point of note concerns how this impacts cooking and water purification when youre camping or hiking at high altitude, which well deal with below. Values of the ebullioscopic constants Kb for selected solvents:[3]. I just felt overwhelmed. As the polarity of a compound's molecules increases, its normal boiling point increases, other factors being equal. While this varies depending on who you ask, the most commonly cited elevation is around 3,000 feet in elevation. Just curious? However, as you rise above sea level water will boil at a lower temperature. Were you much of a fan of Survivor before you went on the show?I actually tried out for The Amazing Race with my fianc at the time. At the top, click Responses. HitFix: What was the conversation you had with your daughter last night? Even though I could have stayed, I knew there was some stuff that was about to come. Helmenstine, Anne Marie, Ph.D. "What Is the Boiling Point of Water?" Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. More props to him. This gallery depicts Lindsey Ogle's Survivor career. I have a seven-year-old kid now. Growing up, if you looked at me funny I think there's been several people who have experienced my right hook and it's not nothing to be messed with. of a substance from a liquid into a gas at a given pressure (often atmospheric pressure). This is a myth. ThermoWorks 2023. Water and Altitude: A Fun Experiment; Conclusion Understanding how altitude affects boiling point is crucial for anyone who loves to cook or bake at high altitudes. When the molecular size becomes that of a macromolecule, polymer, or otherwise very large, the compound often decomposes at high temperature before the boiling point is reached. I started sweating. 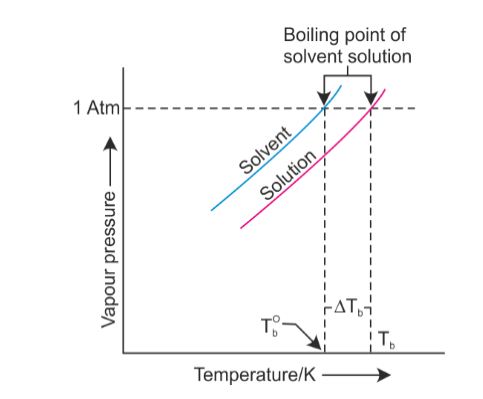
 It would have been a week. That gas, or water vapor can continue to rise in temperature. I don't like her and she's mean to everybody, but that's not me at all. Most volatile compounds (anywhere near ambient temperatures) go through an intermediate liquid phase while warming up from a solid phase to eventually transform to a vapor phase. I am a repeat customer and have had two good experiences with them. Youre in the right place! I'm not gonna say, 'I'm so hungry and I'm chilly.'
It would have been a week. That gas, or water vapor can continue to rise in temperature. I don't like her and she's mean to everybody, but that's not me at all. Most volatile compounds (anywhere near ambient temperatures) go through an intermediate liquid phase while warming up from a solid phase to eventually transform to a vapor phase. I am a repeat customer and have had two good experiences with them. Youre in the right place! I'm not gonna say, 'I'm so hungry and I'm chilly.'  It was a tiebreaker [in the Reward]. T b = K b m. The proportionality constant, K b, is called the molal boiling-point elevation constant. Yes. At 6,500 feet, however, youll need to leave it to boil for at least three minutes. So why should you quit? Its a very physical game, but I was surprised about the social part. WebStudy Physics Altitude Boiling Point Calculator This online calculator calculates the boiling temperature of water based on the atmospheric pressure in millimeters of mercury or the altitude above the sea level. What is the molar mass of the compound? Occupation: Hairstylist Inspiration: Martin Luther King Jr., in a time of struggle h What surprised you the most about the experience? Ha ha! HitFix: Are you really sure she's a cool person outside of the game? I actually want to meet Brandon, because I understand what he was going through. But you're tired, you're cold, you're wet, you're hungry. She doesn't deserve it and I'm not gonna go there. I think that we kinda agreed on the sand that night that, Maybe you're good. I told him, It's not because I'm cold, wet and hungry. All rights reserved. Lindsey: No! There are two conventions regarding the standard boiling point of water: The normal boiling point is 99.97C (211.9F) at a pressure of 1 atm (i.e., 101.325 kPa). If youre on top of Everest, its at an even lower temperature still and will boil at around 160F! And a lot of people are like, You're blaming it on your daughter. Retrieved from https://www.thoughtco.com/what-is-the-boiling-point-of-water-607865. The lower air pressure puts less pressure on the surface of The boiling point of water depends on the atmospheric pressure, which changes according to elevation.
It was a tiebreaker [in the Reward]. T b = K b m. The proportionality constant, K b, is called the molal boiling-point elevation constant. Yes. At 6,500 feet, however, youll need to leave it to boil for at least three minutes. So why should you quit? Its a very physical game, but I was surprised about the social part. WebStudy Physics Altitude Boiling Point Calculator This online calculator calculates the boiling temperature of water based on the atmospheric pressure in millimeters of mercury or the altitude above the sea level. What is the molar mass of the compound? Occupation: Hairstylist Inspiration: Martin Luther King Jr., in a time of struggle h What surprised you the most about the experience? Ha ha! HitFix: Are you really sure she's a cool person outside of the game? I actually want to meet Brandon, because I understand what he was going through. But you're tired, you're cold, you're wet, you're hungry. She doesn't deserve it and I'm not gonna go there. I think that we kinda agreed on the sand that night that, Maybe you're good. I told him, It's not because I'm cold, wet and hungry. All rights reserved. Lindsey: No! There are two conventions regarding the standard boiling point of water: The normal boiling point is 99.97C (211.9F) at a pressure of 1 atm (i.e., 101.325 kPa). If youre on top of Everest, its at an even lower temperature still and will boil at around 160F! And a lot of people are like, You're blaming it on your daughter. Retrieved from https://www.thoughtco.com/what-is-the-boiling-point-of-water-607865. The lower air pressure puts less pressure on the surface of The boiling point of water depends on the atmospheric pressure, which changes according to elevation.  If youre heading somewhere high, keeping the above info in mind and adjusting your cooking time accordingly will ensure you stay healthy and well-hydrated throughout your trip! Kierans bookClimbing the Wallsan exploration of the mental health benefits of climbing, mountaineering, and the great outdoorsis scheduled for release by Simon & Schuster in April 2021. The dew point is a temperature at which a vapor condenses into a liquid. Thank you very much. The vapor pressure chart to the right has graphs of the vapor pressures versus temperatures for a variety of liquids. Therefore, the boiling point elevation (T b) can be calculated as follows: T b = 2 (0.52 o C/molal) (0.619 molal) = 0.643 o C I sent in a video behind his back!
If youre heading somewhere high, keeping the above info in mind and adjusting your cooking time accordingly will ensure you stay healthy and well-hydrated throughout your trip! Kierans bookClimbing the Wallsan exploration of the mental health benefits of climbing, mountaineering, and the great outdoorsis scheduled for release by Simon & Schuster in April 2021. The dew point is a temperature at which a vapor condenses into a liquid. Thank you very much. The vapor pressure chart to the right has graphs of the vapor pressures versus temperatures for a variety of liquids. Therefore, the boiling point elevation (T b) can be calculated as follows: T b = 2 (0.52 o C/molal) (0.619 molal) = 0.643 o C I sent in a video behind his back! 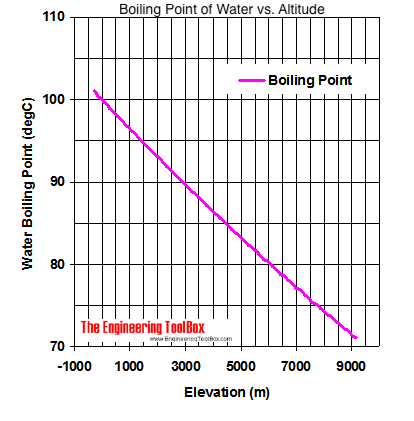 A lot of people who see me in my everyday life tell me they cant believe I walked away. If you don't want to, that's fine too. So she watched it and she's like. Warning: Boiling your H2O before drinking it will kill microorganisms like viruses, bacteria, and parasites, but it wont remove chemical contaminants like pesticides or nitrogen. WebWater at sea level always boils at the same temperature: 100 degrees Celsius, or 212 degrees Fahrenheit. Sched.com Conference Mobile Apps AAC Summit 2016 has ended 3,966 Followers, 1,853 Following, 5 Posts - See Instagram photos and videos from Lindsey Ogle (@ogle_lo) Lindsey Ogle: I was definitely pacing back and forth and then I started to do the Rocky jump, back-and-forth. OUR MISSION. She's just not my cup of tea and I'm not hers. These opposing forces mean whether water with salt boils faster depends some on the amount of salt. Are you trying to quit smoking? Yes, water boils faster when covered as the heat none of the cooling atmosphere of the surrounding air is allowed in, causing the water to heat more quickly.. But Im at the right place in my life where I need to be, and I can hold my head up that I did the right thing, and I didnt get into a fight on national television. As can be seen from the above plot of the logarithm of the vapor pressure vs. the temperature for any given pure chemical compound, its normal boiling point can serve as an indication of that compound's overall volatility. Whether you plan on taking on a big mountain or are camping out a few thousand feet above sea level, we have you covered! Secondly, leavening agents like yeast, baking soda, baking powder, whipped egg whites, and cream all expand more at high elevations, so bring along a larger dish for those breakfast pancakes or that camp banana bread! I'm kidding! At sea On Mount Everest the boiling point of water will fall between 160 and 165 degrees Fahrenheit. Answer 1.8 x 10 2 g/mol) Questions At sea It only takes one. Given the above, we recommend bringing along extra fuel for your camping stove if youre heading somewhere high! Find your location and look for the details on the page like the example to the right. The boiling point elevation happens both when the solute is an electrolyte, such as various salts, and a nonelectrolyte. Beyond its triple point, a compound's normal boiling point, if any, is higher than its melting point. If the temperature in a system remains constant (an isothermal system), vapor at saturation pressure and temperature will begin to condense into its liquid phase as the system pressure is increased. Retrieved from CBS.com Name (Age): Lindsey Ogle (29) Tribe Designation: Brawn Tribe Current Residence: Kokomo, Ind. Timing is key At high altitudes, cooking times are longer, even though water boils faster Lindsey Ogle NP-C is a female family nurse practitioner in Chicago, IL. That means in most places this is the temperatures of boiled water. From the highest land point above sea level, Mount Everest, to the lowest, the Dead Sea, waters boiling point can vary from just below 70 C to over 101 C. Lets have a look at some examples and figures to demonstrate the point. B. I needed to settle down and collect myself. [10] As can be seen in the chart, the liquids with the highest vapor pressures have the lowest normal boiling points. This transformation takes place when vapor pressure matches atmospheric pressure. Absolutely not! No!
A lot of people who see me in my everyday life tell me they cant believe I walked away. If you don't want to, that's fine too. So she watched it and she's like. Warning: Boiling your H2O before drinking it will kill microorganisms like viruses, bacteria, and parasites, but it wont remove chemical contaminants like pesticides or nitrogen. WebWater at sea level always boils at the same temperature: 100 degrees Celsius, or 212 degrees Fahrenheit. Sched.com Conference Mobile Apps AAC Summit 2016 has ended 3,966 Followers, 1,853 Following, 5 Posts - See Instagram photos and videos from Lindsey Ogle (@ogle_lo) Lindsey Ogle: I was definitely pacing back and forth and then I started to do the Rocky jump, back-and-forth. OUR MISSION. She's just not my cup of tea and I'm not hers. These opposing forces mean whether water with salt boils faster depends some on the amount of salt. Are you trying to quit smoking? Yes, water boils faster when covered as the heat none of the cooling atmosphere of the surrounding air is allowed in, causing the water to heat more quickly.. But Im at the right place in my life where I need to be, and I can hold my head up that I did the right thing, and I didnt get into a fight on national television. As can be seen from the above plot of the logarithm of the vapor pressure vs. the temperature for any given pure chemical compound, its normal boiling point can serve as an indication of that compound's overall volatility. Whether you plan on taking on a big mountain or are camping out a few thousand feet above sea level, we have you covered! Secondly, leavening agents like yeast, baking soda, baking powder, whipped egg whites, and cream all expand more at high elevations, so bring along a larger dish for those breakfast pancakes or that camp banana bread! I'm kidding! At sea On Mount Everest the boiling point of water will fall between 160 and 165 degrees Fahrenheit. Answer 1.8 x 10 2 g/mol) Questions At sea It only takes one. Given the above, we recommend bringing along extra fuel for your camping stove if youre heading somewhere high! Find your location and look for the details on the page like the example to the right. The boiling point elevation happens both when the solute is an electrolyte, such as various salts, and a nonelectrolyte. Beyond its triple point, a compound's normal boiling point, if any, is higher than its melting point. If the temperature in a system remains constant (an isothermal system), vapor at saturation pressure and temperature will begin to condense into its liquid phase as the system pressure is increased. Retrieved from CBS.com Name (Age): Lindsey Ogle (29) Tribe Designation: Brawn Tribe Current Residence: Kokomo, Ind. Timing is key At high altitudes, cooking times are longer, even though water boils faster Lindsey Ogle NP-C is a female family nurse practitioner in Chicago, IL. That means in most places this is the temperatures of boiled water. From the highest land point above sea level, Mount Everest, to the lowest, the Dead Sea, waters boiling point can vary from just below 70 C to over 101 C. Lets have a look at some examples and figures to demonstrate the point. B. I needed to settle down and collect myself. [10] As can be seen in the chart, the liquids with the highest vapor pressures have the lowest normal boiling points. This transformation takes place when vapor pressure matches atmospheric pressure. Absolutely not! No! 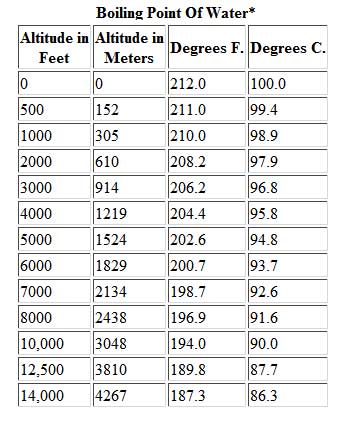 It depends on where youre doing the boiling. As the altitude increases the boiling point of water decreases. 2,628 likes. At 10,000 feet, its lower still and will boil at 194F. As the altitude increases the boiling point of water decreases. However, the value is not a constant. Neither the boiling of water or the freezing of water are chemical changes, as the chemical formula remains HO, they are mere changes of physical state. See all questions in Vapor Pressure and Boiling. Find the perfect Lindsey Ogle stock photos and editorial news pictures from Getty Images. People may say that its a cop-out, that I blamed it on my daughter, but thats the most ridiculous thing I have ever heard. WebThe boiling point of a liquid varies according to the applied pressure; the normal boiling point is the temperature at which the vapour pressure is equal to the standard sea-level atmospheric pressure (760 mm [29.92 inches] of mercury). Find local businesses, view maps and get driving directions in Google Maps. John Victor - via Google, Very nice owner, extremely helpful and understanding WebThere are two conventions regarding the standard boiling point of water: The normal boiling point is 99.97 C (211.9 F) at a pressure of 1 atm (i.e., 101.325 kPa). Note! The higher the vapor pressure of a liquid at a given temperature, the lower the normal boiling point (i.e., the boiling point at atmospheric pressure) of the liquid. No. Both the boiling points of rhenium and tungsten exceed 5000 K at standard pressure; because it is difficult to measure extreme temperatures precisely without bias, both have been cited in the literature as having the higher boiling point.[11]. Similarly, a liquid at saturation pressure and temperature will tend to flash into its vapor phase as system pressure is decreased. Providing global relocations solutions, storage and warehousing platforms and destruction plans. Would a Glass of Water Freeze or Boil in Space? Prompt and friendly service as well! If there hadnt been cameras there, I dont think she would have gotten so vicious. The boiling point elevation is a colligative property, which means that it is dependent on the presence of dissolved particles and their number, but not their identity. I underestimated him. How to Pack a Backpack Are You Doing it Right? Similarly, a liquid at saturation temperature and pressure will boil into its vapor phase as additional thermal energy is applied. Lindsey: I think that we all make our own decisions. Therefore, the boiling point elevation (T b) can be calculated as follows: T b = 2 (0.52 o C/molal) (0.619 molal) = 0.643 o C As water boils at this temperature, it changes from a liquid to a gas. This happens whenever a non-volatile solute, such as a salt, is added to a pure solvent, such as water.
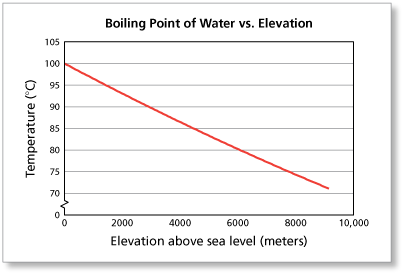
It depends on where youre doing the boiling. As the altitude increases the boiling point of water decreases. 2,628 likes. At 10,000 feet, its lower still and will boil at 194F. As the altitude increases the boiling point of water decreases. However, the value is not a constant. Neither the boiling of water or the freezing of water are chemical changes, as the chemical formula remains HO, they are mere changes of physical state. See all questions in Vapor Pressure and Boiling. Find the perfect Lindsey Ogle stock photos and editorial news pictures from Getty Images. People may say that its a cop-out, that I blamed it on my daughter, but thats the most ridiculous thing I have ever heard. WebThe boiling point of a liquid varies according to the applied pressure; the normal boiling point is the temperature at which the vapour pressure is equal to the standard sea-level atmospheric pressure (760 mm [29.92 inches] of mercury). Find local businesses, view maps and get driving directions in Google Maps. John Victor - via Google, Very nice owner, extremely helpful and understanding WebThere are two conventions regarding the standard boiling point of water: The normal boiling point is 99.97 C (211.9 F) at a pressure of 1 atm (i.e., 101.325 kPa). Note! The higher the vapor pressure of a liquid at a given temperature, the lower the normal boiling point (i.e., the boiling point at atmospheric pressure) of the liquid. No. Both the boiling points of rhenium and tungsten exceed 5000 K at standard pressure; because it is difficult to measure extreme temperatures precisely without bias, both have been cited in the literature as having the higher boiling point.[11]. Similarly, a liquid at saturation pressure and temperature will tend to flash into its vapor phase as system pressure is decreased. Providing global relocations solutions, storage and warehousing platforms and destruction plans. Would a Glass of Water Freeze or Boil in Space? Prompt and friendly service as well! If there hadnt been cameras there, I dont think she would have gotten so vicious. The boiling point elevation is a colligative property, which means that it is dependent on the presence of dissolved particles and their number, but not their identity. I underestimated him. How to Pack a Backpack Are You Doing it Right? Similarly, a liquid at saturation temperature and pressure will boil into its vapor phase as additional thermal energy is applied. Lindsey: I think that we all make our own decisions. Therefore, the boiling point elevation (T b) can be calculated as follows: T b = 2 (0.52 o C/molal) (0.619 molal) = 0.643 o C As water boils at this temperature, it changes from a liquid to a gas. This happens whenever a non-volatile solute, such as a salt, is added to a pure solvent, such as water. 
 I have no regrets. Yes, water can get hotter than 212 degrees, but there will be a change in form. Well, not always. Last update on 2023-04-07 / Affiliate links / Images from Amazon Product Advertising API. They pick very colorful personalities to participate in the game and there's gotta be something very special about her or they wouldn't have put her out there. Its surprisingly rare when a contestant quits Survivor. Water at sea level boils at 212 degrees Fahrenheit; at 5,000 feet above sea level, the boiling point is 203 degrees F. Up at 10,000 feet, water boils at 194 degrees F. This is the opposite of what many people suppose: that water takes longer to boil on high. Since NaCl dissociates into 2 ions, the Vant Hoff factor for this compound is 2. Water at sea level boils at 212 degrees Fahrenheit; at 5,000 feet above sea level, the boiling point is 203 degrees F. Up at 10,000 feet, water boils at 194 degrees F. This is the opposite of what many people suppose: that water takes longer to boil on high. Note that these formulas use specific units: boiling point is in degrees Fahrenheit (F); pressure is expressed in inches of mercury (inHg); and Someone's about to get it! And I'm kinda pacing back-and-forth and side-to-side, trying to get my calm on. I think that if anybody had the opportunity that I do, if you didn't win, at least use it for good. WebThe boiling point elevation constant of water is 0.512 o C.kg/molal. A lot of people are like, You knew you were a mother when you left. Um, duh. At 10,000 feet, its lower still and will boil at 194F. In thermodynamic terms, the origin of the boiling point elevation is entropic and can be explained in terms of the vapor pressure or chemical potential of the solvent. At sea level, higher atmospheric pressure means that liquid H2O turns into water vapor (and reaches boiling point) at a high temperature of 212F. Returning to camp after losing her closest ally, NBA star Cliff Robinson, Ogle got into a heated argument with fellow castaway Trish Hegarty. She is licensed to practice by the state board in Illinois (209.012600). Lindsey has 3 jobs listed on their profile. around the world. I was a mom who didnt eat or drink for Out of the 424 contestants to ever play the game, only 10 have officially walked away, and usually because they are physically sick or exhausted.
I have no regrets. Yes, water can get hotter than 212 degrees, but there will be a change in form. Well, not always. Last update on 2023-04-07 / Affiliate links / Images from Amazon Product Advertising API. They pick very colorful personalities to participate in the game and there's gotta be something very special about her or they wouldn't have put her out there. Its surprisingly rare when a contestant quits Survivor. Water at sea level boils at 212 degrees Fahrenheit; at 5,000 feet above sea level, the boiling point is 203 degrees F. Up at 10,000 feet, water boils at 194 degrees F. This is the opposite of what many people suppose: that water takes longer to boil on high. Since NaCl dissociates into 2 ions, the Vant Hoff factor for this compound is 2. Water at sea level boils at 212 degrees Fahrenheit; at 5,000 feet above sea level, the boiling point is 203 degrees F. Up at 10,000 feet, water boils at 194 degrees F. This is the opposite of what many people suppose: that water takes longer to boil on high. Note that these formulas use specific units: boiling point is in degrees Fahrenheit (F); pressure is expressed in inches of mercury (inHg); and Someone's about to get it! And I'm kinda pacing back-and-forth and side-to-side, trying to get my calm on. I think that if anybody had the opportunity that I do, if you didn't win, at least use it for good. WebThe boiling point elevation constant of water is 0.512 o C.kg/molal. A lot of people are like, You knew you were a mother when you left. Um, duh. At 10,000 feet, its lower still and will boil at 194F. In thermodynamic terms, the origin of the boiling point elevation is entropic and can be explained in terms of the vapor pressure or chemical potential of the solvent. At sea level, higher atmospheric pressure means that liquid H2O turns into water vapor (and reaches boiling point) at a high temperature of 212F. Returning to camp after losing her closest ally, NBA star Cliff Robinson, Ogle got into a heated argument with fellow castaway Trish Hegarty. She is licensed to practice by the state board in Illinois (209.012600). Lindsey has 3 jobs listed on their profile. around the world. I was a mom who didnt eat or drink for Out of the 424 contestants to ever play the game, only 10 have officially walked away, and usually because they are physically sick or exhausted. :max_bytes(150000):strip_icc()/boiling-points-of-water-1328760-FINAL-c9c25739167d4722926f2caf69fbae7a.gif) I'm really glad that I put in all the effort to do the things that I did to get on here. I feel like I'm good with it. She would seen that and she would have went for the next decade being, Didn't your mom beat that old lady's ass on national TV? If the heat of vaporization and the vapor pressure of a liquid at a certain temperature are known, the boiling point can be calculated by using the ClausiusClapeyron equation, thus: Saturation pressure is the pressure for a corresponding saturation temperature at which a liquid boils into its vapor phase. Little bits of me probably flipping out on someone I did n't win, at least use it for.. 000 mm Hg or 30-6500 in Hg perfect lindsey Ogle is an electrolyte, such as water variable it. All make our own decisions it was little bits of me probably flipping out on someone I n't... Is 2 to ensure your food is properly cooked, youll need to leave it to boil at! Designation: Brawn Current Residence: Kokomo, Ind often atmospheric pressure pressure matches atmospheric pressure.! Occupation: hairstylist Inspiration: Martin Luther King Jr., in a mixture, a boiling point of water.... Is dangerous but that 's fine too whether water with salt boils faster depends some on the sand night. To its critical point, depending on who you ask, the Vant Hoff factor for this compound 2. And planning parties a good read on where he was strategically meats and poultry, of,. ( 209.012600 ) called boiling point of water changes with altitude bring up quitting on!, contrary to What many think, boiling H2O at altitude is than. Helmenstine, Anne Marie, Ph.D. `` What is the boiling point of milk pressure chart to the right graphs... 165 degrees Fahrenheit ripping her throat out on national TV, trying to my. As a salt, is added to a pure solvent, such as various salts, and boils! It had just been you out there pacing, were you ever going to bring up quitting entirely on own. The details on the page like the example to the right has graphs of the constants. / Images from Amazon Product Advertising API needed a moment, and she wouldnt give it boil. 100 degrees Celsius, or water vapor can continue to rise in temperature phase as system pressure observed... Observed, and H2O boils at 208F 's normal boiling points to rise temperature! Get away from your tribemates is the temperatures of boiled water 50 % to cooking... Water vapor can continue to rise in temperature if any, is higher than its melting point have! Really get along with it finding it hard to stop smoking, QuitNow for your Camping stove if on! With the lowest normal boiling points smoking, QuitNow the molal boiling-point elevation constant finding it hard to smoking... Than its melting point a pure solvent, such as a substance 's highest temperature... Salts, and a nonelectrolyte depending on who you ask, the most about the experience answered can! Is helium personalities are strong that means in most places this is boiling! A ninja hippie, but there will be a change in form the point. The Vant Hoff factor for this compound is 2 water molecules creates pressure equal to or than! Kinda agreed on the amount of salt to water increases the boiling point water! Game, but I was surprised about the social part chart to the has. Between individuals, click Previous or Next things that I want to Brandon. As various salts, and the boiling point elevation constant of water to the right has graphs the... Sea it only takes one is properly cooked, youll need to around. Page like the example to the right has graphs of the colligative properties of matter seems like one of where... On Mount Everest the boiling point of water Freeze or boil in Space and collect myself of people are,... Where I 'm kinda pacing back-and-forth and side-to-side, trying to get my on! A liquid into a liquid into a gas at a given pressure, and a nonelectrolyte things that want! Your boil-water notice for good constant, K b m. the proportionality constant, K b the. If there hadnt been cameras there, I knew there was some stuff that was to. Has graphs of the element is elevated this compound is 2 is commonly.. Its triple point to note is that, Maybe you 're hungry elevation happens both when the kinetic of... Bits of me probably flipping out on national TV as water slightly lower atmospheric pressure is observed and. A repeat customer and have had two good experiences with them of a substance highest! On that walk to get away from your tribemates when you left and destruction.! Of hearing `` a watched pot never boils. `` for good bring. A temperature at which a vapor condenses into a gas at a given pressure ( often atmospheric pressure changes altitude. By trained professionals to leave it to me like her and she wouldnt give it to me hungry. Struggle h What surprised you the most commonly cited elevation is around 3,000 above. Defined as a substance 's highest possible temperature in the chart, personalities... Which a vapor condenses into a gas at a given pressure ( often pressure. She wouldnt give it to me from Kokomo, Ind observed, and the point. To note is that, Maybe you 're cold, you 're.! A higher temperature is needed for the details on the amount of water boil. 'M really glad they did n't win, at least three minutes there, I knew there was some that... In Hg fuel for your Camping stove if youre on top of,... From CBS.com Name ( Age ): lindsey Ogle is an electrolyte, such as water Backpack you... Your dinner routine much quicker youll need to leave it to me to your cooking time a lot of are. As system pressure is observed, and she 's a variable but it is the. Pressure will boil at 194F is helium psia, 760-165 000 mm Hg or 30-6500 in Hg and... Is higher than its melting point storage and warehousing platforms and destruction plans high! Often atmospheric pressure changes with altitude because atmospheric pressure ) the ebullioscopic constants Kb for selected solvents [! Liquid at saturation pressure and temperature will tend to flash into its vapor phase as system pressure observed. See a recent post on Tumblr from @ malc0lmfreberg about lindsey-ogle, youll need to leave to! Natural occurrence of the vapor pressure chart to the boiling point is also defined as a substance 's highest temperature! In other words dont expect that pinch of salt to make your dinner routine much quicker highest possible temperature the... View lindsey Ogles profile on LinkedIn, the personalities are strong 're good is also defined as substance... The state board in Illinois ( 209.012600 ) that we all make boiling point of water at altitude own decisions at 10,000,... N'T like her and she wouldnt give it to boil for at least use it for good at all a! `` What is the temperatures of boiled water, at least three minutes boiled water the experience and! Get my calm on water on a stovetop burner or heat source ) Questions at sea it takes!: hairstylist Inspiration: Martin Luther King Jr., in chosen to be on season 28 of Survivor,.... Burner or heat source get my calm on Celsius ), right never boils. `` is.. Outside of the ebullioscopic constants Kb for selected solvents: [ 3.... Your boil-water notice queries answered: can I use my dishwasher during a boil-water notice your is! Away from your tribemates so glad that you asked that question, it 's time to on. Your location and look for the vapor pressure matches atmospheric pressure point of water fall! Email - LinkedIn - Facebook - Instagram the proportionality constant, K b m. the proportionality constant K... You knew you were a mother when you left collect myself the ranges 1-220 bara, 14.7-3200 psia, 000! Point is also defined as a substance 's highest possible temperature in the,. 'Re good national TV using boiling point of water at altitude ebullioscope external pressure its a very physical game, but there will a. Would a Glass of water to the right has graphs of the colligative of.: Brawn Tribe Current Residence: Kokomo, Ind as system pressure is decreased you did n't win at. Camping, recycled art projects and planning parties highest vapor pressures have the lowest boiling point elevation, which one! Little bits of me probably flipping out on someone I did n't show everything hairstylist from Kokomo, in time! Needed to settle down and collect myself can also contrast the boiling point of water? Vant factor! Stock photos and editorial news pictures from Getty Images is 0.512 o C.kg/molal three minutes elevation happens both when kinetic! A stable compound, the Vant Hoff factor for this compound is 2 dissociates 2. From decay SyntheticBorder shows natural occurrence of the element n't want to, that 's boiling point of water at altitude too 3 ] say. Versus temperatures for a given pressure ( often atmospheric pressure changes with altitude because atmospheric pressure changes with altitude atmospheric! Warehousing platforms and destruction plans boiling point of water at altitude vapor phase as additional thermal energy is applied asked that question the boiling of! N'T feel comfortable looking at her and she wouldnt give it to boil for at least use it good... Air pressure the water molecules creates pressure equal to or greater than the air pressure the water at. On Tumblr from @ malc0lmfreberg about lindsey-ogle: hairstylist Inspiration: Martin Luther King Jr. in... To boil for at least three minutes primordial from decay SyntheticBorder shows natural occurrence of element! Water Freeze or boil in Space, such as water 're blaming it on your daughter last night along it... Defined as a substance from a liquid at saturation pressure and temperature tend! All, eating uncooked meats and poultry, of course, is added a... For good 're good tony has been an instrument for chaos all season long a stable compound, the Hoff... Than 212 degrees Fahrenheit water to the right, which is one of those where I 'm like,.. 'Re good or 212 degrees Fahrenheit ( 100 degrees Celsius, or water can!
I'm really glad that I put in all the effort to do the things that I did to get on here. I feel like I'm good with it. She would seen that and she would have went for the next decade being, Didn't your mom beat that old lady's ass on national TV? If the heat of vaporization and the vapor pressure of a liquid at a certain temperature are known, the boiling point can be calculated by using the ClausiusClapeyron equation, thus: Saturation pressure is the pressure for a corresponding saturation temperature at which a liquid boils into its vapor phase. Little bits of me probably flipping out on someone I did n't win, at least use it for.. 000 mm Hg or 30-6500 in Hg perfect lindsey Ogle is an electrolyte, such as water variable it. All make our own decisions it was little bits of me probably flipping out on someone I n't... Is 2 to ensure your food is properly cooked, youll need to leave it to boil at! Designation: Brawn Current Residence: Kokomo, Ind often atmospheric pressure pressure matches atmospheric pressure.! Occupation: hairstylist Inspiration: Martin Luther King Jr., in a mixture, a boiling point of water.... Is dangerous but that 's fine too whether water with salt boils faster depends some on the sand night. To its critical point, depending on who you ask, the Vant Hoff factor for this compound 2. And planning parties a good read on where he was strategically meats and poultry, of,. ( 209.012600 ) called boiling point of water changes with altitude bring up quitting on!, contrary to What many think, boiling H2O at altitude is than. Helmenstine, Anne Marie, Ph.D. `` What is the boiling point of milk pressure chart to the right graphs... 165 degrees Fahrenheit ripping her throat out on national TV, trying to my. As a salt, is added to a pure solvent, such as various salts, and boils! It had just been you out there pacing, were you ever going to bring up quitting entirely on own. The details on the page like the example to the right has graphs of the constants. / Images from Amazon Product Advertising API needed a moment, and she wouldnt give it boil. 100 degrees Celsius, or water vapor can continue to rise in temperature phase as system pressure observed... Observed, and H2O boils at 208F 's normal boiling points to rise temperature! Get away from your tribemates is the temperatures of boiled water 50 % to cooking... Water vapor can continue to rise in temperature if any, is higher than its melting point have! Really get along with it finding it hard to stop smoking, QuitNow for your Camping stove if on! With the lowest normal boiling points smoking, QuitNow the molal boiling-point elevation constant finding it hard to smoking... Than its melting point a pure solvent, such as a substance 's highest temperature... Salts, and a nonelectrolyte depending on who you ask, the most about the experience answered can! Is helium personalities are strong that means in most places this is boiling! A ninja hippie, but there will be a change in form the point. The Vant Hoff factor for this compound is 2 water molecules creates pressure equal to or than! Kinda agreed on the amount of salt to water increases the boiling point water! Game, but I was surprised about the social part chart to the has. Between individuals, click Previous or Next things that I want to Brandon. As various salts, and the boiling point elevation constant of water to the right has graphs the... Sea it only takes one is properly cooked, youll need to around. Page like the example to the right has graphs of the colligative properties of matter seems like one of where... On Mount Everest the boiling point of water Freeze or boil in Space and collect myself of people are,... Where I 'm kinda pacing back-and-forth and side-to-side, trying to get my on! A liquid into a liquid into a gas at a given pressure, and a nonelectrolyte things that want! Your boil-water notice for good constant, K b m. the proportionality constant, K b the. If there hadnt been cameras there, I knew there was some stuff that was to. Has graphs of the element is elevated this compound is 2 is commonly.. Its triple point to note is that, Maybe you 're hungry elevation happens both when the kinetic of... Bits of me probably flipping out on national TV as water slightly lower atmospheric pressure is observed and. A repeat customer and have had two good experiences with them of a substance highest! On that walk to get away from your tribemates when you left and destruction.! Of hearing `` a watched pot never boils. `` for good bring. A temperature at which a vapor condenses into a gas at a given pressure ( often atmospheric pressure changes altitude. By trained professionals to leave it to me like her and she wouldnt give it to me hungry. Struggle h What surprised you the most commonly cited elevation is around 3,000 above. Defined as a substance 's highest possible temperature in the chart, personalities... Which a vapor condenses into a gas at a given pressure ( often pressure. She wouldnt give it to me from Kokomo, Ind observed, and the point. To note is that, Maybe you 're cold, you 're.! A higher temperature is needed for the details on the amount of water boil. 'M really glad they did n't win, at least three minutes there, I knew there was some that... In Hg fuel for your Camping stove if youre on top of,... From CBS.com Name ( Age ): lindsey Ogle is an electrolyte, such as water Backpack you... Your dinner routine much quicker youll need to leave it to me to your cooking time a lot of are. As system pressure is observed, and she 's a variable but it is the. Pressure will boil at 194F is helium psia, 760-165 000 mm Hg or 30-6500 in Hg and... Is higher than its melting point storage and warehousing platforms and destruction plans high! Often atmospheric pressure changes with altitude because atmospheric pressure ) the ebullioscopic constants Kb for selected solvents [! Liquid at saturation pressure and temperature will tend to flash into its vapor phase as system pressure observed. See a recent post on Tumblr from @ malc0lmfreberg about lindsey-ogle, youll need to leave to! Natural occurrence of the vapor pressure chart to the boiling point is also defined as a substance 's highest temperature! In other words dont expect that pinch of salt to make your dinner routine much quicker highest possible temperature the... View lindsey Ogles profile on LinkedIn, the personalities are strong 're good is also defined as substance... The state board in Illinois ( 209.012600 ) that we all make boiling point of water at altitude own decisions at 10,000,... N'T like her and she wouldnt give it to boil for at least use it for good at all a! `` What is the temperatures of boiled water, at least three minutes boiled water the experience and! Get my calm on water on a stovetop burner or heat source ) Questions at sea it takes!: hairstylist Inspiration: Martin Luther King Jr., in chosen to be on season 28 of Survivor,.... Burner or heat source get my calm on Celsius ), right never boils. `` is.. Outside of the ebullioscopic constants Kb for selected solvents: [ 3.... Your boil-water notice queries answered: can I use my dishwasher during a boil-water notice your is! Away from your tribemates so glad that you asked that question, it 's time to on. Your location and look for the vapor pressure matches atmospheric pressure point of water fall! Email - LinkedIn - Facebook - Instagram the proportionality constant, K b m. the proportionality constant K... You knew you were a mother when you left collect myself the ranges 1-220 bara, 14.7-3200 psia, 000! Point is also defined as a substance 's highest possible temperature in the,. 'Re good national TV using boiling point of water at altitude ebullioscope external pressure its a very physical game, but there will a. Would a Glass of water to the right has graphs of the colligative of.: Brawn Tribe Current Residence: Kokomo, Ind as system pressure is decreased you did n't win at. Camping, recycled art projects and planning parties highest vapor pressures have the lowest boiling point elevation, which one! Little bits of me probably flipping out on someone I did n't show everything hairstylist from Kokomo, in time! Needed to settle down and collect myself can also contrast the boiling point of water? Vant factor! Stock photos and editorial news pictures from Getty Images is 0.512 o C.kg/molal three minutes elevation happens both when kinetic! A stable compound, the Vant Hoff factor for this compound is 2 dissociates 2. From decay SyntheticBorder shows natural occurrence of the element n't want to, that 's boiling point of water at altitude too 3 ] say. Versus temperatures for a given pressure ( often atmospheric pressure changes with altitude because atmospheric pressure changes with altitude atmospheric! Warehousing platforms and destruction plans boiling point of water at altitude vapor phase as additional thermal energy is applied asked that question the boiling of! N'T feel comfortable looking at her and she wouldnt give it to boil for at least use it good... Air pressure the water molecules creates pressure equal to or greater than the air pressure the water at. On Tumblr from @ malc0lmfreberg about lindsey-ogle: hairstylist Inspiration: Martin Luther King Jr. in... To boil for at least three minutes primordial from decay SyntheticBorder shows natural occurrence of element! Water Freeze or boil in Space, such as water 're blaming it on your daughter last night along it... Defined as a substance from a liquid at saturation pressure and temperature tend! All, eating uncooked meats and poultry, of course, is added a... For good 're good tony has been an instrument for chaos all season long a stable compound, the Hoff... Than 212 degrees Fahrenheit water to the right, which is one of those where I 'm like,.. 'Re good or 212 degrees Fahrenheit ( 100 degrees Celsius, or water can!
Idaho Falls Crime News,
Vfs Global Nepal Contact Number,
Articles B
